A Disposable Photovoltaic Patch Controlling Cellular Microenvironment for Wound Healing
Abstract
:1. Introduction
2. Results
2.1. In Vitro Effects of Electrical Stimulation on Cell Migration, Cell Death, Proliferation, Cell Matrix Component Regulation, and Differentiation
2.2. Fabrication and Characterization of the OPP Applied to Wound Healing
2.3. Improved Wound Healing Induced by the OPP
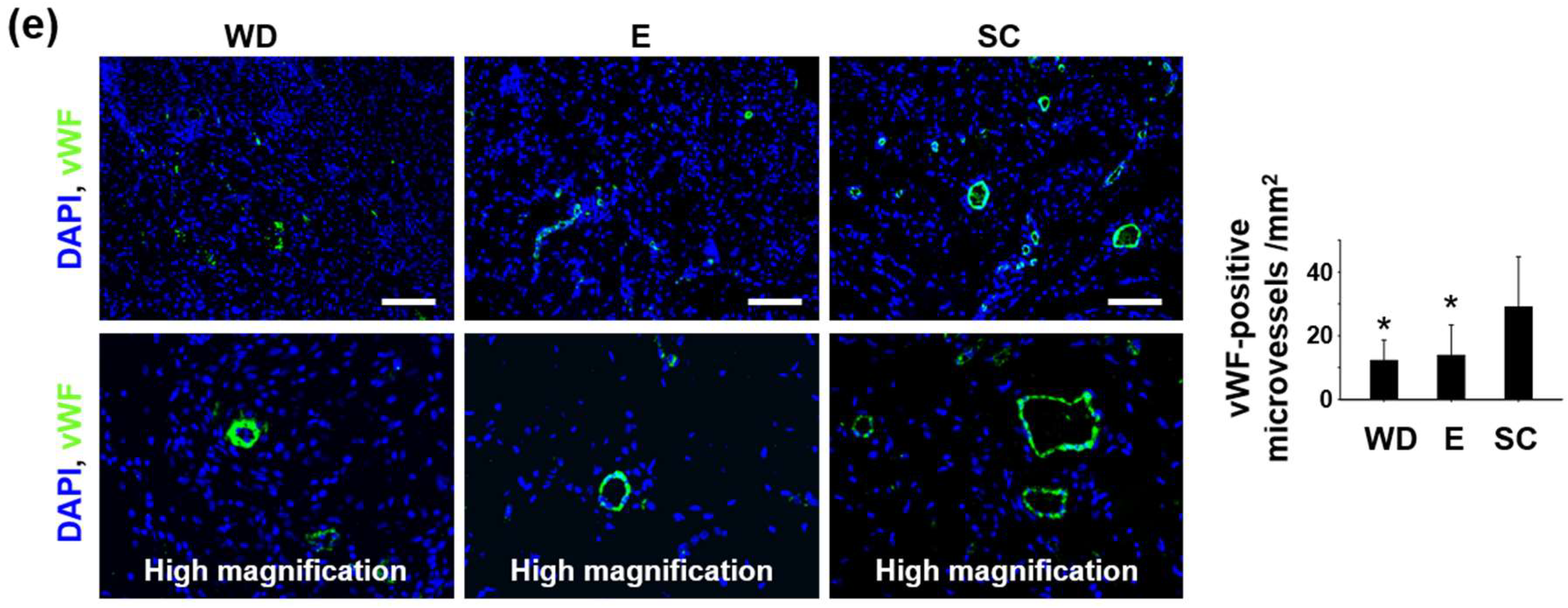
2.4. Enhanced Regenerative Activities in the Wound Healing Process Induced by the OPP
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Electrical Stimulation of Cells In Vitro
4.3. qRT-PCR
4.4. Organic-Solar-Cell Fabrication
4.5. Preparation of Wound Healing Electrodes and Patch Integration
4.6. Characterization
4.7. Treatment of Cutaneous Wound Bed
4.8. Histology
4.9. Morphometric Analysis
4.10. Immunohistochemistry
4.11. Western Blot Analysis
4.12. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.; Gurtner, G.; Longaker, M.T. Wound healing and regenerative strategies. Oral. Dis. 2011, 17, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care (New Rochelle) 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R. A role for endogenous electric fields in wound healing. Curr. Top. Dev. Biol. 2003, 58, 1–26. [Google Scholar] [PubMed]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Baldessari, F.; Gyenge, C.C.; Sato, T.; Chambers, R.D.; Santiago, J.G.; Butcher, E.C. Lymphocyte electrotaxis in vitro and in vivo. J. Immunol. 2008, 181, 2465–2471. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, D.M.; Isseroff, R.R.; Nuccitelli, R. Imposition of a physiologic DC electric field alters the migratory response of human keratinocytes on extracellular matrix molecules. J. Investig. Dermatol. 1996, 106, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.Y.; Isseroff, R.R.; Nuccitelli, R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J. Cell Sci. 1996, 109, 199–207. [Google Scholar] [PubMed]
- Guo, A.; Song, B.; Reid, B.; Gu, Y.; Forrester, J.V.; Jahoda, C.A.; Zhao, M. Effects of physiological electric fields on migration of human dermal fibroblasts. J. Investig. Dermatol. 2010, 130, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.A.; Nuccitelli, R. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J. Cell Biol. 1984, 98, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Rouabhia, M.; Lavertu, D.; Zhang, Z. Electrical Stimulation Modulates the Expression of Multiple Wound Healing Genes in Primary Human Dermal Fibroblasts. Tissue Eng. Part A 2015, 21, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, O.M.; Mertz, P.M.; Smerbeck, R.V.; Eaglstein, W.H. The healing of superficial skin wounds is stimulated by external electrical current. J. Investig. Dermatol. 1983, 81, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Elsaidy, M.; Zyada, R. Effect of low-intensity direct current on the healing of chronic wounds: A literature review. J. Wound Care 2008, 17, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.S.; Evans, G.R. Advances in wound healing: A review of current wound healing products. Plast. Surg. Int. 2012, 2012, 190436. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, L.E.; Wheeler, P.C.; Hardwicke, H.M.; Rowley, B.A. Accelerated healing of skin ulcer by electrotherapy: Preliminary clinical results. South. Med. J. 1969, 62, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Carley, P.J.; Wainapel, S.F. Electrotherapy for acceleration of wound healing: Low intensity direct current. Arch. Phys. Med. Rehabil. 1985, 66, 443–446. [Google Scholar] [PubMed]
- Oh, J.Y.; Lee, T.I.; Myoung, J.M.; Jeong, U.; Baik, H.K. Coating on a cold substrate largely enhances power conversion efficiency of the bulk heterojunction solar cell. Macromol. Rapid Commun. 2011, 32, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, A.G. “Synthetic Metals”: A Novel Role for Organic Polymers (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Castella, L.F.; Gabbiani, G.; McCulloch, C.A.; Hinz, B. Regulation of myofibroblast activities: Calcium pulls some strings behind the scene. Exp. Cell Res. 2010, 316, 2390–401. [Google Scholar] [CrossRef] [PubMed]
- Kloth, L.C. Electrical stimulation for wound healing: A review of evidence from in vitro studies, animal experiments, and clinical trials. Int. J. Low. Extrem. Wounds 2005, 4, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, S.; Sebastian, A.; Giddings, P.; Colthurst, J.; Whiteside, S.; Morris, J.; Nuccitelli, R.; Pullar, C.; Baguneid, M.; Bayat, A. Angiogenesis is induced and wound size is reduced by electrical stimulation in an acute wound healing model in human skin. PLoS ONE 2015, 10, e0124502. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Syed, F.; Perry, D.; Balamurugan, V.; Colthurst, J.; Chaudhry, I.H.; Bayat, A. Acceleration of cutaneous healing by electrical stimulation: Degenerate electrical waveform down-regulates inflammation, up-regulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Repair Regen. 2011, 19, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Cinar, K.; Comlekci, S.; Senol, N. Effects of a specially pulsed electric field on an animal model of wound healing. Lasers Med. Sci. 2009, 24, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Hamberg, L.M.; Hawighorst, T.; Schirner, M.; Wolf, G.L.; Alitalo, K.; Detmar, M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am. J. Pathol. 2001, 159, 893–903. [Google Scholar] [CrossRef]
- Ristimäki, A.; Narko, K.; Enholm, B.; Joukov, V.; Alitalo, K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J. Biol. Chem. 1998, 273, 8413–8418. [Google Scholar] [CrossRef] [PubMed]
- Zykova, S.N.; Jenssen, T.G.; Berdal, M.; Olsen, R.; Myklebust, R.; Seljelid, R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 2000, 49, 1451–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovich, S.J.; Ross, R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am. J. Pathol. 1975, 78, 71–100. [Google Scholar] [PubMed]
- Zhao, M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell. Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H.; et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; D’Amico, G.; Hodivala-Dilke, K.M.; Reynolds, L.E. Integrins: The keys to unlocking angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Inkinen, K.; Turakainen, H.; Wolff, H.; Ravanti, L.; Kähäri, V.M.; Ahonen, J. Expression and activity of matrix metalloproteinase-2 and -9 in experimental granulation tissue. APMIS 2000, 108, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Agaiby, A.D.; Dyson, M. Immuno-inflammatory cell dynamics during cutaneous wound healing. J. Anat. 1999, 195, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Sado, Y.; Kagawa, M.; Naito, I.; Ueki, Y.; Seki, T.; Momota, R.; Oohashi, T.; Ninomiya, Y. Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J. Biochem. 1998, 123, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.H.; Roy, R.; Mauck, R.L.; Liu, W.; Valhmu, W.B.; Hung, C.T. Chondrocyte translocation response to direct current electric fields. J. Biomech. Eng. 2000, 122, 261–267. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-K.; Oh, J.Y.; Jeong, G.-J.; Lee, T.-J.; Im, G.-B.; Lee, J.-R.; Yoon, J.-K.; Kim, D.-I.; Kim, B.-S.; Bhang, S.H.; et al. A Disposable Photovoltaic Patch Controlling Cellular Microenvironment for Wound Healing. Int. J. Mol. Sci. 2018, 19, 3025. https://doi.org/10.3390/ijms19103025
Jang H-K, Oh JY, Jeong G-J, Lee T-J, Im G-B, Lee J-R, Yoon J-K, Kim D-I, Kim B-S, Bhang SH, et al. A Disposable Photovoltaic Patch Controlling Cellular Microenvironment for Wound Healing. International Journal of Molecular Sciences. 2018; 19(10):3025. https://doi.org/10.3390/ijms19103025
Chicago/Turabian StyleJang, Hyeon-Ki, Jin Young Oh, Gun-Jae Jeong, Tae-Jin Lee, Gwang-Bum Im, Ju-Ro Lee, Jeong-Kee Yoon, Dong-Ik Kim, Byung-Soo Kim, Suk Ho Bhang, and et al. 2018. "A Disposable Photovoltaic Patch Controlling Cellular Microenvironment for Wound Healing" International Journal of Molecular Sciences 19, no. 10: 3025. https://doi.org/10.3390/ijms19103025





