Voltage-Dependent Sarcolemmal Ion Channel Abnormalities in the Dystrophin-Deficient Heart
Abstract
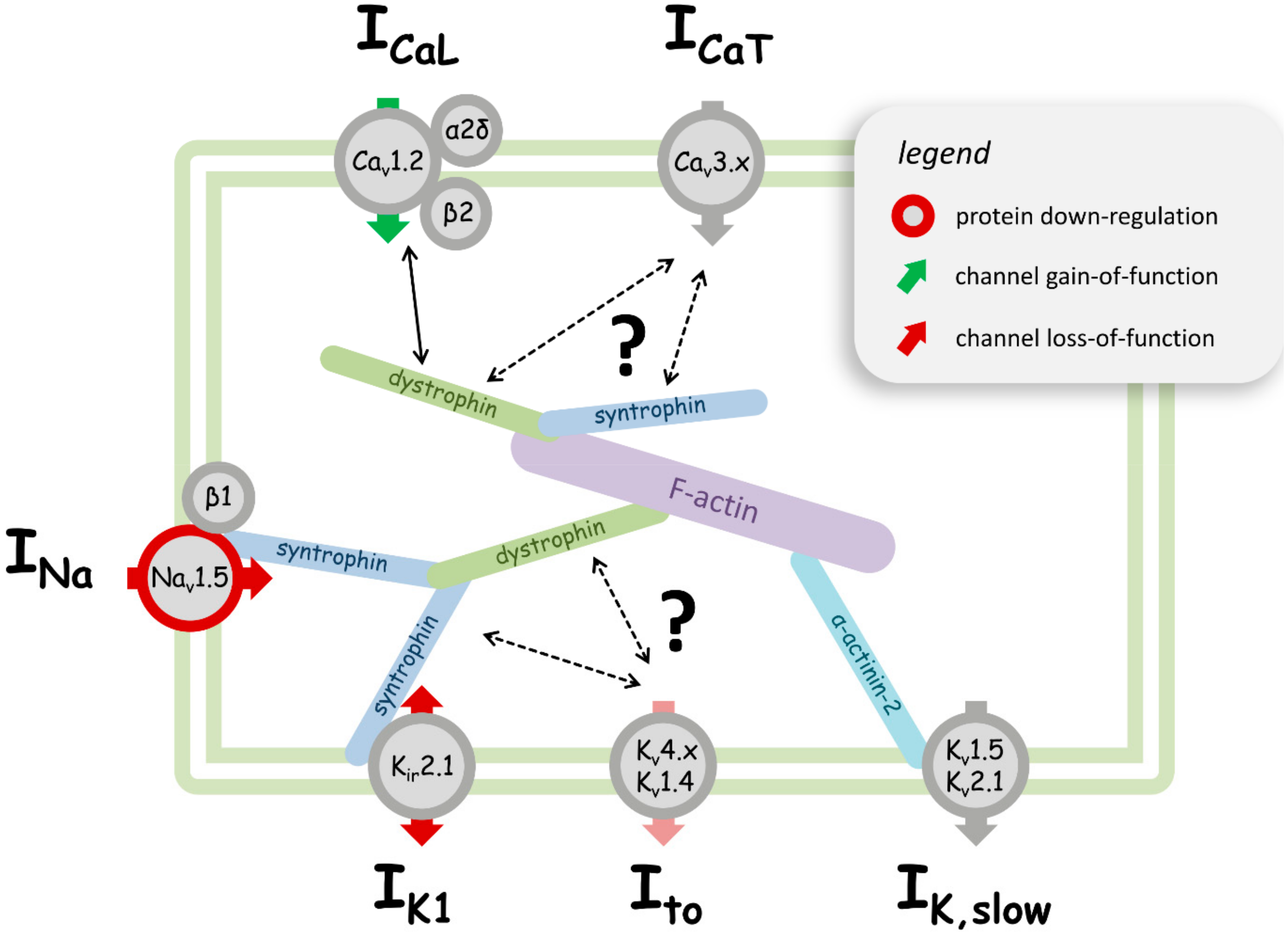
:1. Introduction
2. Dystrophic Cardiac Ion Channel Abnormalities: Evidence from Animal Model Studies
2.1. Sodium Channels
2.2. Calcium Channels
2.3. Potassium Channels
2.4. Ion Channel Abnormalities Prior to Dystrophic Cardiomyopathy Development
3. Dystrophic Cardiac Ion Channel Abnormalities: Evidence from Human Studies
4. Limitations of the Experimental Studies
5. Conclusions, Clinical Implications, and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wallace, G.Q.; McNally, E.M. Mechanisms of Muscle Degeneration, Regeneration, and Repair in the Muscular Dystrophies. Annu. Rev. Physiol. 2009, 71, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, J.M.; Campbell, K.P. Membrane Organization of the Dystrophin-Glycoprotein Complex. Cell 1991, 66, 1121–1131. [Google Scholar] [CrossRef]
- Ervasti, J.M.; Campbell, K.P. A Role for the Dystrophin-Glycoprotein Complex as a Transmembrane Linker between Laminin and Actin. J. Cell Biol. 1993, 122, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Petrof, B.J.; Shragert, J.B.; Stedmant, H.H.; Kellyt, A.M.; Sweeney, H.L. Dystrophin Protects the Sarcolemma from Stresses Developed during Muscle Contraction (Muscular Dystrophy/Muscle Injury/Mdx Mouse). Med. Sci. 1993, 90, 3710–3714. [Google Scholar]
- Bushby, K.M.; Thambyayah, M.; Gardner-Medwin, D. Prevalence and Incidence of Becker Muscular Dystrophy. Lancet 1991, 337, 1022–1024. [Google Scholar] [CrossRef]
- Finsterer, J.; Stöllberger, C. Cardiac Involvement in Becker Muscular Dystrophy. Can. J. Cardiol. 2008, 24, 786–792. [Google Scholar] [CrossRef]
- Ho, R.; Nguyen, M.-L.; Mather, P. Cardiomyopathy in Becker Muscular Dystrophy: Overview. World J. Cardiol. 2016, 8, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Alderton, J.M.; Steinhardt, R.A. How Calcium Influx through Calcium Leak Channels Is Responsible for the Elevated Levels of Calcium-Dependent Proteolysis in Dystrophic Myotubes. Trends Cardiovasc. Med. 2000, 10, 268–272. [Google Scholar] [CrossRef]
- Johnstone, V.P.A.; Viola, H.M.; Hool, L.C. Dystrophic Cardiomyopathy—Potential Role of Calcium in Pathogenesis, Treatment and Novel Therapies. Genes 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Van Westering, T.; Betts, C.; Wood, M. Current Understanding of Molecular Pathology and Treatment of Cardiomyopathy in Duchenne Muscular Dystrophy. Molecules 2015, 20, 8823–8855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenard, A.A.; Becane, H.M.; Tertrain, F.; De Kermadec, J.M.; Weiss, Y.A. Ventricular Arrhythmia in Duchenne Muscular Dystrophy: Prevalence, Significance and Prognosis. Neuromuscul. Disord. 1993, 3, 201–206. [Google Scholar] [CrossRef]
- Tsuda, T.; Fitzgerald, K.K. Dystrophic Cardiomyopathy: Complex Pathobiological Processes to Generate Clinical Phenotype. J. Cardiovasc. Dev. Dis. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, F.; Garry, D.J. Dystrophin-Deficient Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, R.W.; Allen, H.D.; Montanaro, F. Current Understanding and Management of Dilated Cardiomyopathy in Duchenne and Becker Muscular Dystrophy. J. Am. Acad. Nurse Pract. 2009, 21, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S. Cardiac Involvement in Duchenne and Becker Muscular Dystrophy. World J. Cardiol. 2015, 7, 410. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; MacLeod, H. Therapy Insight: Cardiovascular Complications Associated with Muscular Dystrophies. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Verhaert, D.; Richards, K.; Rafael-Fortney, J.A.; Raman, S.V. Cardiac Involvement in Patients with Muscular Dystrophies: Magnetic Resonance Imaging Phenotype and Genotypic Considerations. Circ. Cardiovasc. Imaging 2011, 4, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.Y.; Allen, H.D.; Kim, J.J.; Valdes, S.O.; Wang, Y.; Pignatelli, R.H.; Lotze, T.E.; Miyake, C.Y. Relation of Cardiac Dysfunction to Rhythm Abnormalities in Patients with Duchenne or Becker Muscular Dystrophies. Am. J. Cardiol. 2016, 117, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Melacini, P.; Fanin, M.; Danieli, G.A.; Villanova, C.; Martinello, F.; Miorin, M.; Freda, M.P.; Miorelli, M.; Mostacciuolo, M.L.; Fasoli, G.; et al. Myocardial Involvement Is Very Frequent among Patients Affected with Subclinical Becker’s Muscular Dystrophy. Circulation 1996, 94, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.P.; Ray, S.G. Cardiac Involvement in Muscular Dystrophies. QJM 2008, 101, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, M.; Fujimoto, A.; Ueyama, H.; Nishida, Y.; Imamura, S.; Uchino, M.; Ando, Y. Life-Threatening Arrhythmias in a Becker Muscular Dystrophy Family Due to the Duplication of Exons 3-4 of the Dystrophin Gene. Intern. Med. 2015, 54, 3075–3078. [Google Scholar] [CrossRef] [PubMed]
- Florian, A.; Rösch, S.; Bietenbeck, M.; Engelen, M.; Stypmann, J.; Waltenberger, J.; Sechtem, U.; Yilmaz, A. Cardiac Involvement in Female Duchenne and Becker Muscular Dystrophy Carriers in Comparison to Their First-Degree Male Relatives: A Comparative Cardiovascular Magnetic Resonance Study. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Politano, L.; Nigro, V.; Nigro, G.; Petretta, V.R.; Passamano, L.; Papparella, S.; Di Somma, S.; Comi, L.I. Development of Cardiomyopathy in Female Carriers of Duchenne and Becker Muscular Dystrophies. JAMA 1996, 275, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Judge, D.P.; Kass, D.A.; Thompson, W.R.; Wagner, K.R. Pathophysiology and Therapy of Cardiac Dysfunction in Duchenne Muscular Dystrophy. Am. J. Cardiovasc. Drugs 2011, 11, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ather, S.; Wang, W.; Wang, Q.; Li, N.; Anderson, M.E.; Wehrens, X.H.T. Inhibition of CaMKII Phosphorylation of RyR2 Prevents Inducible Ventricular Arrhythmias in Mice with Duchenne Muscular Dystrophy. Heart Rhythm 2013, 10, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; Mcdonald, C.; Pandya, S.; et al. Review Diagnosis and Management of Duchenne Muscular Dystrophy, Part 2: Implementation of Multidisciplinary Care. Lancet Neurol. 2010, 9, 177–189. [Google Scholar] [CrossRef]
- Sadeghi, A.; Doyle, A.D.; Johnson, B.D. Regulation of the Cardiac L-Type Ca2+ Channel by the Actin-Binding Proteins Alpha-Actinin and Dystrophin. Am. J. Physiol. Cell Physiol. 2002, 282, C1502–C1511. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Strege, P.; Miller, S.M.; Makielski, J.; Ackerman, M.; Gibbons, S.J.; Farrugia, G. Syntrophin Γ2 Regulates SCN5A Gating by a PDZ Domain-Mediated Interaction. J. Biol. Chem. 2003, 278, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Ponce-Balbuena, D.; Jalife, J. Protein Assemblies of Sodium and Inward Rectifier Potassium Channels Control Cardiac Excitability and Arrhythmogenesis. Am. J. Physiol. Circ. Physiol. 2015, 308, H1463–H1473. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, C.Y.; Taniguti, A.P.T.; Pertille, A.; Neto, H.S.; Marques, M.J. Stretch-Activated Calcium Channel Protein TRPC1 Is Correlated with the Different Degrees of the Dystrophic Phenotype in Mdx Mice. Am. J. Physiol. Physiol. 2011, 301, C1344–C1350. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Kim, G.E.; Holewinski, R.J.; Venkatraman, V.; Zhu, G.; Bedja, D.; Kass, D.A.; Van Eyk, J.E. Transient Receptor Potential Channel 6 Regulates Abnormal Cardiac S-Nitrosylation in Duchenne Muscular Dystrophy. Proc. Natl. Acad. Sci. USA 2017, 114, E10763–E10771. [Google Scholar] [CrossRef] [PubMed]
- Graciotti, L.; Becker, J.; Granata, A.L.; Procopio, A.D.; Tessarollo, L.; Fulgenzi, G. Dystrophin Is Required for the Normal Function of the Cardio-Protective KATP Channel in Cardiomyocytes. PLoS ONE 2011, 6, e27034. [Google Scholar] [CrossRef] [PubMed]
- Koenig, X.; Dysek, S.; Kimbacher, S.; Mike, A.K.; Cervenka, R.; Lukacs, P.; Nagl, K.; Dang, X.B.; Todt, H.; Bittner, R.E.; et al. Voltage-Gated Ion Channel Dysfunction Precedes Cardiomyopathy Development in the Dystrophic Heart. PLoS ONE 2011, 6, e20300. [Google Scholar] [CrossRef] [PubMed]
- Gavillet, B.; Rougier, J.S.; Domenighetti, A.A.; Behar, R.; Boixel, C.; Ruchat, P.; Lehr, H.A.; Pedrazzini, T.; Abriel, H. Cardiac Sodium Channel Nav1.5 Is Regulated by a Multiprotein Complex Composed of Syntrophins and Dystrophin. Circ. Res. 2006, 99, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Albesa, M.; Ogrodnik, J.; Rougier, J.-S.; Abriel, H. Regulation of the Cardiac Sodium Channel Nav1.5 by Utrophin in Dystrophin-Deficient Mice. Cardiovasc. Res. 2011, 89, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Rougier, J.-S.; Gavillet, B.; Abriel, H. Proteasome Inhibitor (MG132) Rescues Nav1.5 Protein Content and the Cardiac Sodium Current in Dystrophin-Deficient Mdx5cv Mice. Front. Physiol. 2013, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Sicinski, P.; Geng, Y.; Ryder-Cook, A.S.; Barnard, E.A.; Darlison, M.G.; Barnard, P.J. The Molecular Basis of Muscular Dystrophy in the Mdx Mouse: A Point Mutation. Science 1989, 244, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Im, W.B.; Phelps, S.F.; Copen, E.H.; Adams, E.G.; Slightom, J.L.; Chamberlain, J.S. Differential Expression of Dystrophin Isoforms in Strains of Mdx Mice with Different Mutations. Hum. Mol. Genet. 1996, 5, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, A.E.; Rafael, J.A.; Skinner, J.A.; Brown, S.C.; Potter, A.C.; Metzinger, L.; Watt, D.J.; Dickson, J.G.; Tinsley, J.M.; Davies, K.E. Utrophin-Dystrophin-Deficient Mice as a Model for Duchenne Muscular Dystrophy. Cell 1997, 90, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Grady, R.M.; Teng, H.; Nichol, M.C.; Cunningham, J.C.; Wilkinson, R.S.; Sanes, J.R. Skeletal and Cardiac Myopathies in Mice Lacking Utrophin and Dystrophin: A Model for Duchenne Muscular Dystrophy. Cell 1997, 90, 729–738. [Google Scholar] [CrossRef]
- Colussi, C.; Berni, R.; Rosati, J.; Straino, S.; Vitale, S.; Spallotta, F.; Baruffi, S.; Bocchi, L.; Delucchi, F.; Rossi, S.; et al. The Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid Reduces Cardiac Arrhythmias in Dystrophic Mice. Cardiovasc. Res. 2010, 87, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Zhang, X.; Li, J.; Ai, X.; Zhang, L.; Yu, D.; Ge, S.; Peng, Y.; Chen, X. Blunted Cardiac Beta-Adrenergic Response as an Early Indication of Cardiac Dysfunction in Duchenne Muscular Dystrophy. Cardiovasc. Res. 2014, 103, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Prins, K.W.; Asp, M.L.; Zhang, H.; Wang, W.; Metzger, J.M. Microtubule-Mediated Misregulation of Junctophilin-2 Underlies T-Tubule Disruptions and Calcium Mishandling in Mdx Mice. JACC Basic Transl. Sci. 2016, 1, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.M.; Adams, A.M.; Davies, S.M.K.; Fletcher, S.; Filipovska, A.; Hool, L.C. Impaired Functional Communication between the L-Type Calcium Channel and Mitochondria Contributes to Metabolic Inhibition in the Mdx Heart. Proc. Natl. Acad. Sci. USA 2014, 111, E2905–E2914. [Google Scholar] [CrossRef] [PubMed]
- Lohan, J.; Culligan, K.; Ohlendieck, K. Deficiency in Cardiac Dystrophin Affects the Abundance of the alpha-/beta-Dystroglycan Complex. J. Biomed. Biotechnol. 2005, 2005, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rubi, L.; Koenig, X.; Kubista, H.; Todt, H.; Hilber, K. Decreased Inward Rectifier Potassium Current IK1 in Dystrophin-Deficient Ventricular Cardiomyocytes. Channels 2017, 11, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Alloatti, G.; Gallo, M.P.; Penna, C.; Levi, R.C. Properties of Cardiac Cells from Dystrophic Mouse. J. Mol. Cell. Cardiol. 1995, 27, 1775–1779. [Google Scholar] [CrossRef]
- Ullrich, N.D.; Fanchaouy, M.; Gusev, K.; Shirokova, N.; Niggli, E. Hypersensitivity of Excitation-Contraction Coupling in Dystrophic Cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1992–H2003. [Google Scholar] [CrossRef] [PubMed]
- Koenig, X.; Rubi, L.; Obermair, G.J.; Cervenka, R.; Dang, X.B.; Lukacs, P.; Kummer, S.; Bittner, R.E.; Kubista, H.; Todt, H.; et al. Enhanced Currents through L-Type Calcium Channels in Cardiomyocytes Disturb the Electrophysiology of the Dystrophic Heart. AJP Heart Circ. Physiol. 2014, 306, H564–H573. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.M.; Davies, S.M.K.; Filipovska, A.; Hool, L.C. L-Type Ca2+ Channel Contributes to Alterations in Mitochondrial Calcium Handling in the Mdx Ventricular Myocyte. Am. J. Physiol. Circ. Physiol. 2013, 304, H767–H775. [Google Scholar] [CrossRef] [PubMed]
- Woolf, P.J.; Lu, S.; Cornford-Nairn, R.; Watson, M.; Xiao, X.-H.; Holroyd, S.M.; Brown, L.; Hoey, A.J. Alterations in Dihydropyridine Receptors in Dystrophin-Deficient Cardiac Muscle. Am. J. Physiol. Circ. Physiol. 2006, 290, H2439–H2445. [Google Scholar] [CrossRef] [PubMed]
- Rubi, L.; Todt, H.; Kubista, H.; Koenig, X.; Hilber, K. Calcium Current Properties in Dystrophin-deficient Ventricular Cardiomyocytes from Aged Mdx Mice. Physiol. Rep. 2018, 6, e13567. [Google Scholar] [CrossRef] [PubMed]
- Pacioretty, L.M.; Cooper, B.J.; Gilmour, R.F. Reduction of the Transient Outward Potassium Current in Canine X-Linked Muscular Dystrophy. Circulation 1994, 90, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Shy, D.; Gillet, L.; Ogrodnik, J.; Albesa, M.; Verkerk, A.O.; Wolswinkel, R.; Rougier, J.-S.; Barc, J.; Essers, M.C.; Syam, N.; et al. PDZ Domain-Binding Motif Regulates Cardiomyocyte Compartment-Specific NaV1.5 Channel Expression and Function. Circulation 2014, 130, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, S.; Zmoos, A.-F.; Ogrodnik, J.; Balse, E.; Raad, N.; El-Haou, S.; Albesa, M.; Bittihn, P.; Luther, S.; Lehnart, S.E.; et al. SAP97 and Dystrophin Macromolecular Complexes Determine Two Pools of Cardiac Sodium Channels Nav1.5 in Cardiomyocytes. Circ. Res. 2011, 108, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Bostick, B.; Yue, Y.; Lai, Y.; Long, C.; Li, D.; Duan, D. Adeno-Associated Virus Serotype-9 Microdystrophin Gene Therapy Ameliorates Electrocardiographic Abnormalities in Mdx Mice. Hum. Gene Ther. 2008, 19, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Greally, E.; Davison, B.J.; Blain, A.; Laval, S.; Blamire, A.; Straub, V.; MacGowan, G.A. Heterogeneous Abnormalities of In-Vivo Left Ventricular Calcium Influx and Function in Mouse Models of Muscular Dystrophy Cardiomyopathy. J. Cardiovasc. Magn. Reson. 2013, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.; Daly, M.; Chamberlain, J.S.; Metzger, J.M. Age-Dependent Dystrophin Loss and Genetic Reconstitution Establish a Molecular Link between Dystrophin and Heart Performance during Aging. Mol. Ther. 2011, 19, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Kuwahara, K.; Takano, M.; Arai, Y.; Kuwabara, Y.; Yasuno, S.; Nakagawa, Y.; Nakanishi, M.; Harada, M.; Fujiwara, M.; et al. T-Type Ca2+ Channel Blockade Prevents Sudden Death in Mice With Heart Failure. Circulation 2009, 120, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Vassort, G.; Talavera, K.; Alvarez, J. Role of T-Type Ca2+ Channels in the Heart. Cell Calcium 2006, 40, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, O.; von Wegner, F.; Chamberlain, J.S.; Fink, R.H.A.; Rohrbach, P. L-Type Ca2+ Channel Function Is Linked to Dystrophin Expression in Mammalian Muscle. PLoS ONE 2008, 3, e1762. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.D.; Emrick, M.A.; Sadilek, M.; Scheuer, T.; Catterall, W.A. Molecular Mechanism of Calcium Channel Regulation in the Fight-or-Flight Response. Sci. Signal. 2010, 3, ra70. [Google Scholar] [CrossRef] [PubMed]
- Cserne Szappanos, H.; Muralidharan, P.; Ingley, E.; Petereit, J.; Millar, A.H.; Hool, L.C. Identification of a Novel CAMP Dependent Protein Kinase A Phosphorylation Site on the Human Cardiac Calcium Channel. Sci. Rep. 2017, 7, 15118. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Oz, S.; Benmocha, A.; Dascal, N. Regulation of Cardiac L-Type Ca2+ Channel CaV1.2 via the β-Adrenergic-CAMP-Protein Kinase a Pathway: Old Dogmas, Advances, and New Uncertainties. Circ. Res. 2013, 113, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Oz, S.; Pankonien, I.; Belkacemi, A.; Flockerzi, V.; Klussmann, E.; Haase, H.; Dascal, N. Protein Kinase A Regulates C-Terminally Truncated CaV 1.2 in Xenopus Oocytes: Roles of N- and C-Termini of the α1C Subunit. J. Physiol. 2017, 595, 3181–3202. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Li, N.; van Oort, R.J.; Reynolds, C.; Skapura, D.G.; Wehrens, X.H.T. Genetic Inhibition of PKA Phosphorylation of RyR2 Prevents Dystrophic Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2010, 107, 13165–13170. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.E.; Bryant, S.M.; Ashley, E.A.; Lygate, C.A.; Rakovic, S.; Wallis, H.L.; Neubauer, S.; Terrar, D.A.; Casadei, B. Cardiac Neuronal Nitric Oxide Synthase Isoform Regulates Myocardial Contraction and Calcium Handling. Circ. Res. 2003, 92, e52–e59. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.N.; Duglan, D.; Casadei, B.; Carnicer, R. Nitric Oxide Synthase Regulation of Cardiac Excitation–contraction Coupling in Health and Disease. J. Mol. Cell. Cardiol. 2014, 73, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Bia, B.L.; Cassidy, P.J.; Young, M.E.; Rafael, J.A.; Leighton, B.; Davies, K.E.; Radda, G.K.; Clarke, K. Decreased Myocardial NNOS, Increased INOS and Abnormal ECGs in Mouse Models of Duchenne Muscular Dystrophy. J. Mol. Cell. Cardiol. 1999, 31, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, J.; Schneider, J.S.; Crassous, P.-A.; Zheng, R.; Gonzalez, J.P.; Xie, L.-H.; Beuve, A.; Fraidenraich, D.; Peluffo, R.D. Nitric Oxide Signalling Pathway in Duchenne Muscular Dystrophy Mice: Up-Regulation of L-Arginine Transporters. Biochem. J. 2013, 449, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.P.; Crassous, P.-A.; Schneider, J.S.; Beuve, A.; Fraidenraich, D. Neuronal Nitric Oxide Synthase Localizes to Utrophin Expressing Intercalated Discs and Stabilizes Their Structural Integrity. Neuromuscul. Disord. 2015, 25, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, R.J.; Wood, M.J.; Davies, K.E. Therapy for Duchenne Muscular Dystrophy: Renewed Optimism from Genetic Approaches. Nat. Rev. Genet. 2013, 14, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.E.; Lu, X.; Lei, M.; Xiang, F.-L.; Hammoud, L.; Jiang, M.; Wang, H.; Jones, D.L.; Sims, S.M.; Feng, Q. Neuronal Nitric Oxide Synthase Protects Against Myocardial Infarction-Induced Ventricular Arrhythmia and Mortality in Mice. Circulation 2009, 120, 1345–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Youm, J.B.; Jin, C.Z.; Shin, D.H.; Zhao, Z.H.; Seo, E.Y.; Jang, J.H.; Kim, S.J.; Jin, Z.H.; Zhang, Y.H. Modulation of L-Type Ca2+ Channel Activity by Neuronal Nitric Oxide Synthase and Myofilament Ca2+ Sensitivity in Cardiac Myocytes from Hypertensive Rat. Cell Calcium 2015, 58, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Barouch, L.A.; Harrison, R.W.; Skaf, M.W.; Rosas, G.O.; Cappola, T.P.; Kobeissi, Z.A.; Hobai, I.A.; Lemmon, C.A.; Burnett, A.L.; O’Rourke, B.; et al. Nitric Oxide Regulates the Heart by Spatial Confinement of Nitric Oxide Synthase Isoforms. Nature 2002, 416, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Abi-Gerges, N.; Fischmeister, R.; Méry, P.F. G Protein-Mediated Inhibitory Effect of a Nitric Oxide Donor on the L-Type Ca2+ Current in Rat Ventricular Myocytes. J. Physiol. 2001, 531, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.R.; Treuer, A.V.; Lamirault, G.; Mayo, V.; Cao, Y.; Dulce, R.A.; Hare, J.M. NADPH Oxidase-2 Inhibition Restores Contractility and Intracellular Calcium Handling and Reduces Arrhythmogenicity in Dystrophic Cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H710–H721. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Cserne Szappanos, H.; Ingley, E.; Hool, L.C. The Cardiac L-Type Calcium Channel Alpha Subunit Is a Target for Direct Redox Modification during Oxidative Stress-the Role of Cysteine Residues in the Alpha Interacting Domain. Clin. Exp. Pharmacol. Physiol. 2017, 44, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Cserne Szappanos, H.; Viola, H.; Hool, L.C. L-Type Calcium Channel: Clarifying the “Oxygen Sensing Hypothesis”. Int. J. Biochem. Cell Biol. 2017, 86, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Viola, H.M.; Filipovska, A.; Hool, L.C. Cav1.2 Calcium Channel Is Glutathionylated during Oxidative Stress in Guinea Pig and Ischemic Human Heart. Free Radic. Biol. Med. 2011, 51, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.M.; Arthur, P.G.; Hool, L.C. Transient Exposure to Hydrogen Peroxide Causes an Increase in Mitochondria-Derived Superoxide as a Result of Sustained Alteration in L-Type Ca2+ Channel Function in the Absence of Apoptosis in Ventricular Myocytes. Circ. Res. 2007, 100, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.A.; Allen, D.G. The Role of Reactive Oxygen Species in the Hearts of Dystrophin-Deficient Mdx Mice. Am. J. Physiol. Circ. Physiol. 2007, 293, H1969–H1977. [Google Scholar] [CrossRef] [PubMed]
- Nanni, S.; Re, A.; Ripoli, C.; Gowran, A.; Nigro, P.; D’Amario, D.; Amodeo, A.; Crea, F.; Grassi, C.; Pontecorvi, A.; et al. The Nuclear Pore Protein Nup153 Associates with Chromatin and Regulates Cardiac Gene Expression in Dystrophic Mdx Hearts. Cardiovasc. Res. 2016, 112, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Barrie, E.S.; Smith, R.M.; Sanford, J.C.; Sadee, W. MRNA Transcript Diversity Creates New Opportunities for Pharmacological Intervention. Mol. Pharmacol. 2012, 81, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Koivumäki, J.T.; Takalo, J.; Korhonen, T.; Tavi, P.; Weckström, M. Modelling Sarcoplasmic Reticulum Calcium ATPase and Its Regulation in Cardiac Myocytes. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 2181–2202. [Google Scholar] [CrossRef] [PubMed]
- Moise, N.S.; Valentine, B.A.; Brown, C.A.; Erb, H.N.; Beck, K.A.; Cooper, B.J.; Gilmour, R.F. Duchenne’s Cardiomyopathy in a Canine Model: Electrocardiographic and Echocardiographic Studies. J. Am. Coll. Cardiol. 1991, 17, 812–820. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Ramachandran, J.; Himelman, E.; Badr, M.A.; Kang, C.; Nouet, J.; Fefelova, N.; Xie, L.-H.; Shirokova, N.; Contreras, J.E.; et al. Normalization of Connexin 43 Protein Levels Prevents Cellular and Functional Signs of Dystrophic Cardiomyopathy in Mice. Neuromuscul. Disord. 2018, 28, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, J.; Thireau, J.; Reiken, S.; Cassan, C.; Richard, S.; Matecki, S.; Marks, A.R.; Lacampagne, A. Leaky RyR2 Trigger Ventricular Arrhythmias in Duchenne Muscular Dystrophy. Proc. Natl. Acad. Sci. USA 2010, 107, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Lakatta, E.G.; Xiao, R.P. Age-Associated Alterations in Calcium Current and Its Modulation in Cardiac Myocytes. Drugs Aging 1998, 13, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.A.; Allen, D.G. Intracellular Calcium Handling in Ventricular Myocytes from Mdx Mice. Am. J. Physiol. Circ. Physiol. 2007, 292, H846–H855. [Google Scholar] [CrossRef] [PubMed]
- Mijares, A.; Altamirano, F.; Kolster, J.; Adams, J.A.; López, J.R. Age-Dependent Changes in Diastolic Ca2+ and Na+ Concentrations in Dystrophic Cardiomyopathy: Role of Ca2+ Entry and IP3. Biochem. Biophys. Res. Commun. 2014, 452, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.M.; Hool, L.C. Role of the Cytoskeleton in Communication between L-Type Ca2+ Channels and Mitochondria. Clin. Exp. Pharmacol. Physiol. 2013, 40, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Jockusch, H.; Ohlendieck, K. Proteomic Profiling of the Dystrophin-Deficient MDX Heart Reveals Drastically Altered Levels of Key Metabolic and Contractile Proteins. J. Biomed. Biotechnol. 2010, 2010, 648501. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Dowling, P.; Zweyer, M.; Mundegar, R.R.; Henry, M.; Meleady, P.; Swandulla, D.; Ohlendieck, K. Proteomic Analysis of Dystrophin Deficiency and Associated Changes in the Aged Mdx-4cv Heart Model of Dystrophinopathy-Related Cardiomyopathy. J. Proteom. 2016, 145, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, A.N.; Nichols, C.G. Inward Rectifiers in the Heart: An Update on I(K1). J. Mol. Cell. Cardiol. 2001, 33, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Leonoudakis, D.; Conti, L.R.; Anderson, S.; Radeke, C.M.; McGuire, L.M.M.; Adams, M.E.; Froehner, S.C.; Yates, J.R.; Vandenberg, C.A. Protein Trafficking and Anchoring Complexes Revealed by Proteomic Analysis of Inward Rectifier Potassium Channel (Kir2.x)-Associated Proteins. J. Biol. Chem. 2004, 279, 22331–22346. [Google Scholar] [CrossRef] [PubMed]
- Aiba, T.; Tomaselli, G.F. Electrical Remodeling in the Failing Heart. Curr. Opin. Cardiol. 2010, 25, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, J.G.; Hahn, H.S.; Wong, B.L.; Lorenz, J.N.; Wenisch, A.S.; Levin, L.S. Evolution of the Mdx Mouse Cardiomyopathy: Physiological and Morphological Findings. Neuromuscul. Disord. 2004, 14, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Au, C.G.; Butler, T.L.; Sherwood, M.C.; Egan, J.R.; North, K.N.; Winlaw, D.S. Increased Connective Tissue Growth Factor Associated with Cardiac Fibrosis in the Mdx Mouse Model of Dystrophic Cardiomyopathy. Int. J. Exp. Pathol. 2011, 92, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Burelle, Y.; Khairallah, M.; Ascah, A.; Allen, B.G.; Deschepper, C.F.; Petrof, B.J.; Des Rosiers, C. Alterations in Mitochondrial Function as a Harbinger of Cardiomyopathy: Lessons from the Dystrophic Heart. J. Mol. Cell. Cardiol. 2010, 48, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Vatta, M.; Faulkner, G. Cytoskeletal Basis of Ion Channel Function in Cardiac Muscle. Future Cardiol. 2006, 2, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Abriel, H.; Rougier, J.-S.; Jalife, J. Ion Channel Macromolecular Complexes in Cardiomyocytes: Roles in Sudden Cardiac Death. Circ. Res. 2015, 116, 1971–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesse, M.; Kondo, C.S.; Clark, R.B.; Su, L.; Allen, F.L.; Geary-Joo, C.T.M.; Kunnathu, S.; Severson, D.L.; Nygren, A.; Giles, W.R.; et al. Dilated Cardiomyopathy Is Associated with Reduced Expression of the Cardiac Sodium Channel Scn5a. Cardiovasc. Res. 2007, 75, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Houser, S.R.; Margulies, K.B.; Murphy, A.M.; Spinale, F.G.; Francis, G.S.; Prabhu, S.D.; Rockman, H.A.; Kass, D.A.; Molkentin, J.D.; Sussman, M.A.; et al. Animal Models of Heart Failure: A Scientific Statement from the American Heart Association. Circ. Res. 2012, 111, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Li, Y.; Han, L.; Kaplan, A.D.; Ao, Y.; Kalra, S.; Bett, G.C.L.; Rasmusson, R.L.; Denning, C.; Yang, L. Modeling and Study of the Mechanism of Dilated Cardiomyopathy Using Induced Pluripotent Stem Cells Derived from Individuals with Duchenne Muscular Dystrophy. Dis. Model. Mech. 2015, 8, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Mack, D.L.; Moreno, C.M.; Strande, J.L.; Mathieu, J.; Shi, Y.; Markert, C.D.; Wang, Z.; Liu, G.; Lawlor, M.W.; et al. Dystrophin-Deficient Cardiomyocytes Derived from Human Urine: New Biologic Reagents for Drug Discovery. Stem Cell Res. 2014, 12, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Perloff, J.K. Cardiac Rhythm and Conduction in Duchenne’s Muscular Dystrophy: A Prospective Study of 20 Patients. J. Am. Coll. Cardiol. 1984, 3, 1263–1268. [Google Scholar] [CrossRef]
- Yotsukura, M.; Miyagawa, M.; Tsuya, T.; Ishihara, T.; Ishikawa, K. A 10-Year Follow-up Study by Orthogonal Frank Lead ECG on Patients with Progressive Muscular Dystrophy of the Duchenne Type. J. Electrocardiol. 1992, 25, 345–353. [Google Scholar] [CrossRef]
- Xiao, H.B.; Roy, C.; Fujimoto, S.; Gibson, D.G. Natural History of Abnormal Conduction and Its Relation to Prognosis in Patients with Dilated Cardiomyopathy. Int. J. Cardiol. 1996, 53, 163–170. [Google Scholar] [CrossRef]
- Spurney, C.F. Cardiomyopathy of Duchenne Muscular Dystrophy: Current Understanding and Future Directions. Muscle Nerve 2011, 44, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Steare, S.E.; Dubowitz, V.; Benatar, A. Subclinical Cardiomyopathy in Becker Muscular Dystrophy. Br. Heart J. 1992, 68, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Ergul, Y.; Ekici, B.; Nisli, K.; Tatli, B.; Binboga, F.; Acar, G.; Ozmen, M.; Omeroglu, R.E. Evaluation of the North Star Ambulatory Assessment Scale and Cardiac Abnormalities in Ambulant Boys with Duchenne Muscular Dystrophy. J. Paediatr. Child Health 2012, 48, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Nigro, G.; Politano, L.; Santangelo, L.; Petretta, V.R.; Passamano, L.; Panico, F.; De Luca, F.; Montefusco, A.; Comi, L.I. Is the Value of QT Dispersion a Valid Method to Foresee the Risk of Sudden Death? A Study in Becker Patients. Heart 2002, 87, 156–157. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Kinnett, K.; Wang, Y.; Ittenbach, R.F.; Benson, D.W.; Cripe, L. Electrocardiographic Abnormalities in Very Young Duchenne Muscular Dystrophy Patients Precede the Onset of Cardiac Dysfunction. Neuromuscul. Disord. 2011, 21, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, G.; Kondratev, D.; Dyachenko, V.; Kazanski, V.; Gallitelli, M.F. Isolated Cardiomyocytes: Mechanosensitivity of Action Potential, Membrane Current and Ion Concentration; Academia Publishing House Ltd.: Moscow, Russia, 2005. [Google Scholar]
- Allen, D.G.; Whitehead, N.P.; Yeung, E.W. Mechanisms of Stretch-Induced Muscle Damage in Normal and Dystrophic Muscle: Role of Ionic Changes. J. Physiol. 2005, 567, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Rainer, P.P.; Lee, D.-I.; Hao, S.; Bedja, D.; Birnbaumer, L.; Cingolani, O.H.; Kass, D.A. Hyperactive Adverse Mechanical Stress Responses in Dystrophic Heart Are Coupled to Transient Receptor Potential Canonical 6 and Blocked by CGMP-Protein Kinase G Modulation. Circ. Res. 2014, 114, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.F.; Tsien, R.W. The Timothy Syndrome Mutation Differentially Affects Voltage- and Calcium-Dependent Inactivation of CaV1.2 L-Type Calcium Channels. Proc. Natl. Acad. Sci. USA 2008, 105, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.E.; Cheng, E.P.; Mercado, J.L.; Santana, L.F. L-Type Ca2+ Channel Function during Timothy Syndrome. Trends Cardiovasc. Med. 2012, 22, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Splawski, I.; Timothy, K.W.; Sharpe, L.M.; Decher, N.; Kumar, P.; Bloise, R.; Napolitano, C.; Schwartz, P.J.; Joseph, R.M.; Condouris, K.; et al. Ca(V)1.2 Calcium Channel Dysfunction Causes a Multisystem Disorder Including Arrhythmia and Autism. Cell 2004, 119, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Muth, J.N.; Bodi, I.; Lewis, W.; Varadi, G.; Schwartz, A. A Ca2+-Dependent Transgenic Model of Cardiac Hypertrophy: A Role for Protein Kinase Calpha. Circulation 2001, 103, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Sui, T.; Lau, Y.S.; Liu, D.; Liu, T.; Xu, L.; Gao, Y.; Lai, L.; Li, Z.; Han, R. A Novel Rabbit Model of Duchenne Muscular Dystrophy Generated by CRISPR/Cas9. Dis. Model. Mech. 2018, 11, dmm032201. [Google Scholar] [CrossRef] [PubMed]
- Milani-Nejad, N.; Janssen, P.M.L. Small and Large Animal Models in Cardiac Contraction Research: Advantages and Disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Nerbonne, J.M.; Nichols, C.G.; Schwarz, T.L.; Escande, D. Genetic Manipulation of Cardiac K+ Channel Function in Mice: What Have We Learned, and Where Do We Go from Here? Circ. Res. 2001, 89, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Bedada, F.B.; Wheelwright, M.; Metzger, J.M. Maturation Status of Sarcomere Structure and Function in Human IPSC-Derived Cardiac Myocytes. Biochim. Biophys. Acta 2016, 1863, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Du, D.T.M.; Hellen, N.; Kane, C.; Terracciano, C.M.N. Action Potential Morphology of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Does Not Predict Cardiac Chamber Specificity and Is Dependent on Cell Density. Biophys. J. 2015, 108, 1–4. [Google Scholar] [CrossRef] [PubMed]
| (A) Protein Data | |||||
|---|---|---|---|---|---|
| Current | Ion Channel | Gene Name | Change | DMD Model | Reference |
| INa | Nav1.5 | Scn5a | ↓ | mdx, mdx-utr | [35] |
| ↓ | mdx | [41] | |||
| ↓ | mdx5cv | [34] | |||
| ↓ | mdx5cv | [36] | |||
| β1 subunit | Scn1b | na | |||
| ICaL | Cav1.2 | Cacna1c | = | mdx5cv | [34] |
| = | mdx | [42] | |||
| = | mdx | [43] | |||
| = | mdx | [44] | |||
| β2 subunit | Cacnb2 | = | mdx | [42] | |
| α2δ1 subunit | Cacna2d1 | = | mdx | [45] | |
| ICaT | Cav3.1 | Cacna1g | na | ||
| Cav3.2 | Cacna1h | na | |||
| IK1 | Kir2.1 | Kcnj2 | ↓ | mdx5cv | [34] |
| = | mdx, mdx-utr | [46] | |||
| Ito | Kv4.2 | Kcnd2 | na | ||
| Kv4.3 | Kcnd3 | na | |||
| Kv1.4 | Kcna4 | na | |||
| IK,slow | Kv1.5 | Kcna5 | na | ||
| Kv2.1 | Kcnb1 | na | |||
| (B) Functional Data | |||||
| Current | Ion Channel | Gene Name | Change | DMD Model | Reference |
| INa | Nav1.5 | Scn5a | ↓ | mdx, mdx-utr | [35] |
| ↓ | mdx5cv | [34] | |||
| ↓ | mdx, mdx-utr | [33] | |||
| ↓ | mdx5cv | [36] | |||
| ICaL | Cav1.2 | Cacna1c | = | mdx | [47] |
| = | mdx | [48] | |||
| ↑ | mdx, mdx-utr | [33] | |||
| ↑ | mdx, mdx-utr | [49] | |||
| ↑ | mdx | [42] | |||
| ↑ | mdx | [27] | |||
| ↑ | mdx | [50] | |||
| ↑ | mdx | [44] | |||
| ↑ | mdx | [51] | |||
| ICaT | Cav3.1, Cav3.2 | Cacna1g, Cacna1h | = | mdx | [52] |
| IK1 | Kir2.1 | Kcnj2 | ↓ | mdx | [46] |
| Ito | Kv4.2, Kv4.3, Kv1.4 | Kcnd2, Kcnd3, Kcna4 | = | mdx | [47] |
| ↓ | xmd dog | [53] | |||
| IK,slow | Kv1.5, Kv2.1 | Kcna5, Kcnb1 | = | mdx, mdx-utr | [49] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koenig, X.; Ebner, J.; Hilber, K. Voltage-Dependent Sarcolemmal Ion Channel Abnormalities in the Dystrophin-Deficient Heart. Int. J. Mol. Sci. 2018, 19, 3296. https://doi.org/10.3390/ijms19113296
Koenig X, Ebner J, Hilber K. Voltage-Dependent Sarcolemmal Ion Channel Abnormalities in the Dystrophin-Deficient Heart. International Journal of Molecular Sciences. 2018; 19(11):3296. https://doi.org/10.3390/ijms19113296
Chicago/Turabian StyleKoenig, Xaver, Janine Ebner, and Karlheinz Hilber. 2018. "Voltage-Dependent Sarcolemmal Ion Channel Abnormalities in the Dystrophin-Deficient Heart" International Journal of Molecular Sciences 19, no. 11: 3296. https://doi.org/10.3390/ijms19113296




