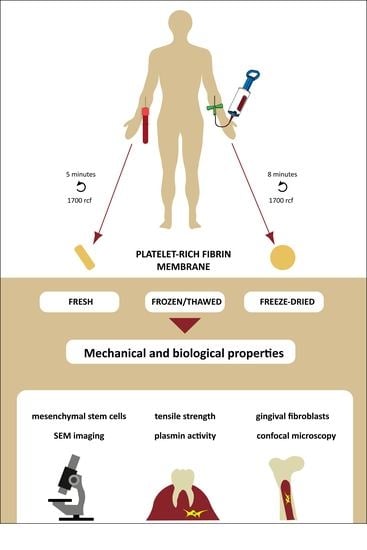
Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Platelet-Rich Fibrin Membrane Preparation
2.2. Sample Preparation
3. Evaluation of Mechanical and Structural Properties
3.1. Tensile Strength Measurements
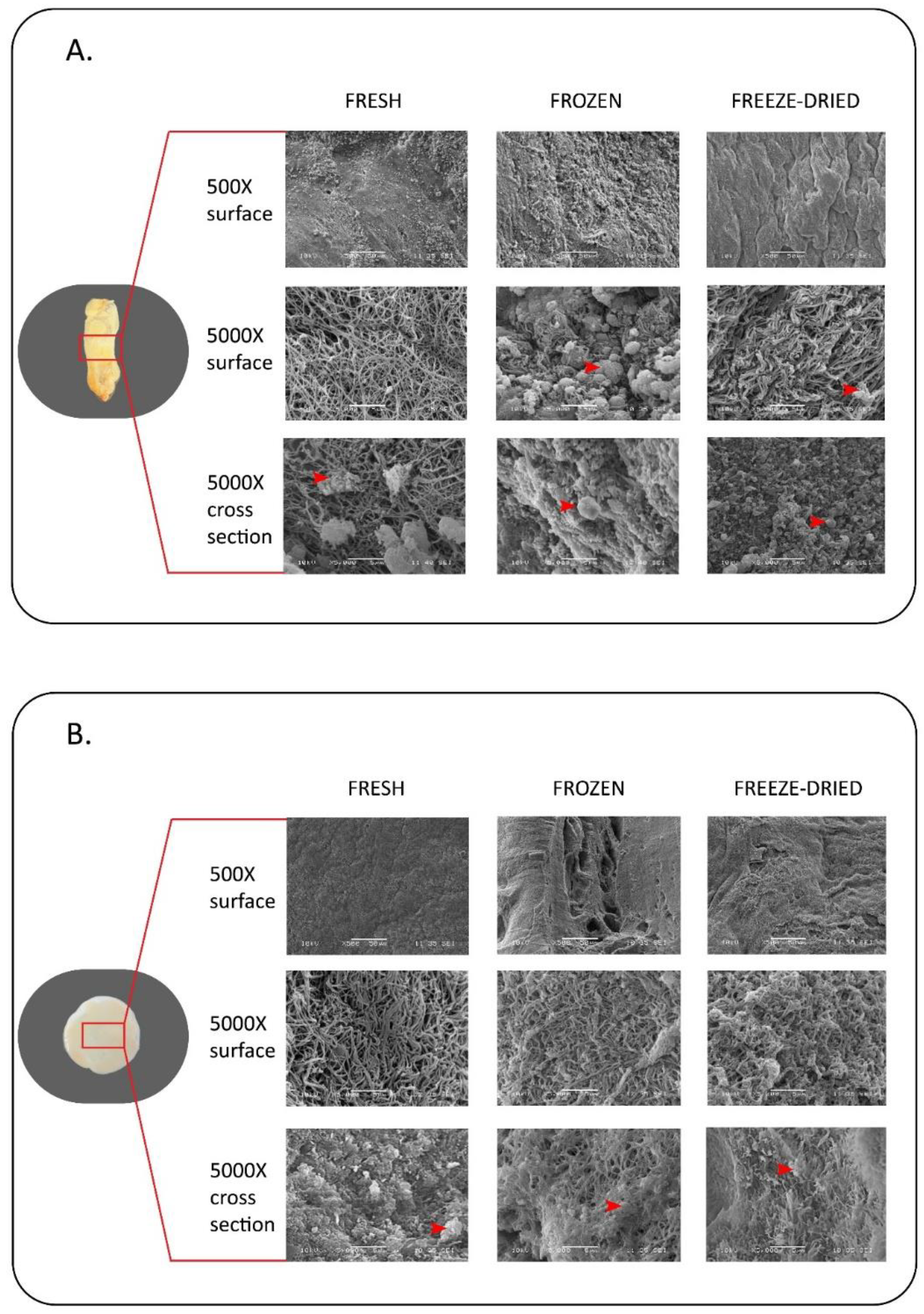
3.2. Scanning Electron Microscopic Observation
4. Evaluation of Biological Properties
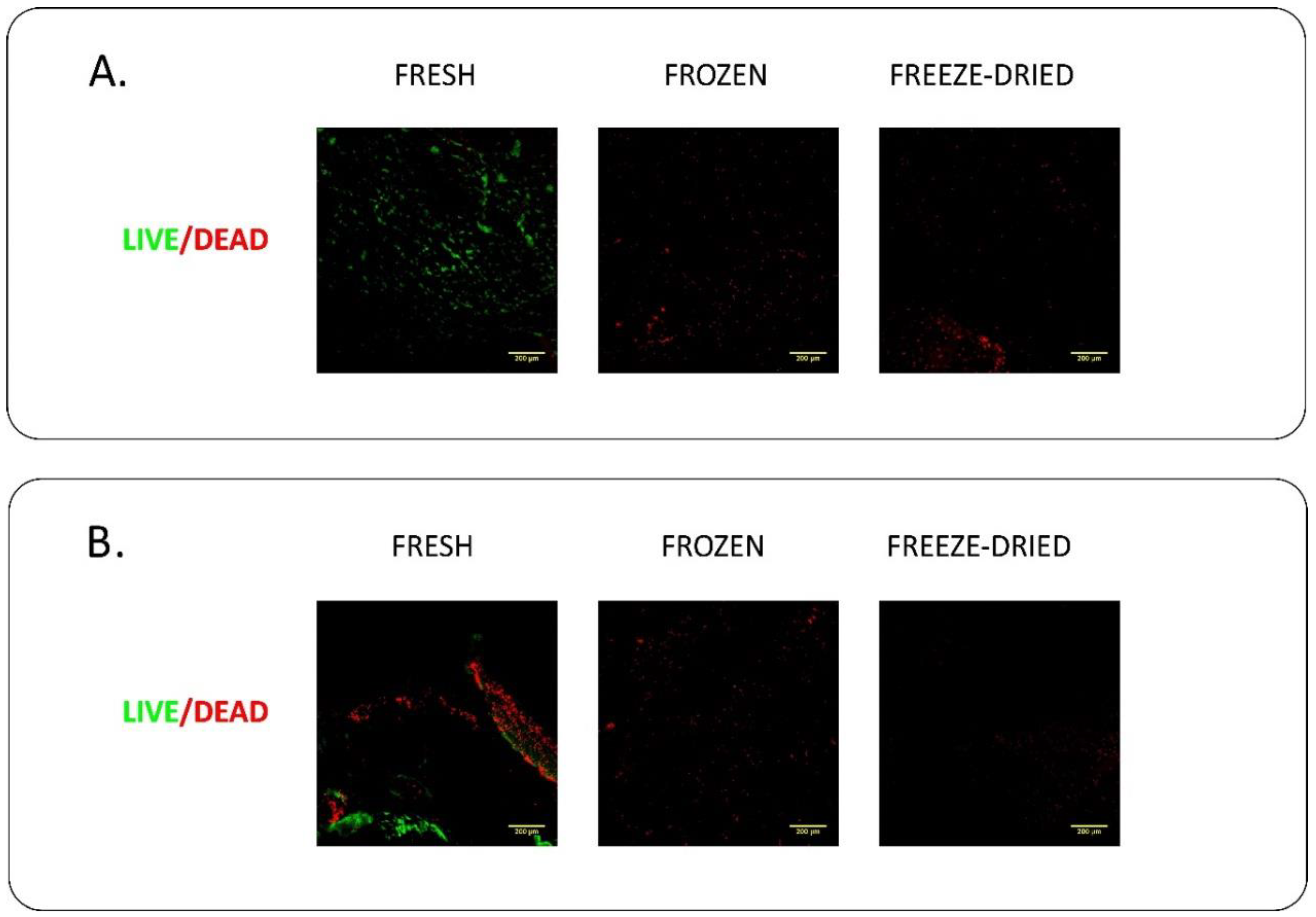
4.1. Live/Dead Staining
4.2. Mesenchymal Stem Cell Culture on PRF Membranes
4.3. Gingival Fibroblast Culture
4.4. Plasmin Activity Measurement
5. Statistical Analysis
6. Results
6.1. Evaluation of Mechanical and Structural Properties
6.2. Evaluation of Biological Properties
7. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Saluja, H.; Dehane, V.; Mahindra, U. Platelet-Rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann. Maxillofac. Surg. 2011, 1, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Duregger, K.; Gable, A.; Eblenkamp, M. Development and evaluation of a spray applicator for platelet-rich plasma. Colloids Surf. B Biointerfaces 2018, 171, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.; Karunakar, P.; Jayadev, M.; Marshal, V.R. Role of Platelet rich fibrin in wound healing: A critical review. J. Conserv. Dent. JCD 2013, 16, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hua, S.; Yang, T.; Ma, J.; Yu, W.; Chen, X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp. Ther. Med. 2018, 15, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.W.; Maloney, J.C.; Archer, R.A.; Brown, K.L.; Mayger, K.; Bromidge, E.S.; Najafi, M.F. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br. J. Biomed. Sci. 2017, 74, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Badis, D.; Omar, B. The effectiveness of platelet-rich plasma on the skin wound healing process: A comparative experimental study in sheep. Vet. World 2018, 11, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yuan, J.; Aisaiti, A.; Liu, Y.; Zhao, J. The effect of platelet-rich plasma on clinical outcomes of the surgical treatment of periodontal intrabony defects: A systematic review and meta-analysis. BMC Oral Health 2016, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Yang, S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016, 4, 16036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Flace, P.; Girolamo, F.; Tarullo, A.; Laino, L.; et al. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: An useful aid in healing and regeneration of bone tissue. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1222–1226. [Google Scholar] [PubMed]
- Kobayashi, E.; Fujioka-Kobayashi, M.; Sculean, A.; Chappuis, V.; Buser, D.; Schaller, B.; Dori, F.; Miron, R.J. Effects of platelet rich plasma (PRP) on human gingival fibroblast, osteoblast and periodontal ligament cell behaviour. BMC Oral Health 2017, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Chicharro-Alcantara, D.; Rubio-Zaragoza, M.; Damia-Gimenez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Pelaez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Khiste, S.V.; Naik Tari, R. Platelet-Rich Fibrin as a Biofuel for Tissue Regeneration. ISRN Biomater. 2013, 2013, 627367. [Google Scholar] [CrossRef]
- Theys, T.; Van Hoylandt, A.; Broeckx, C.E.; Van Gerven, L.; Jonkergouw, J.; Quirynen, M.; van Loon, J. Plasma-rich fibrin in neurosurgery: A feasibility study. Acta Neurochir. 2018. [Google Scholar] [CrossRef] [PubMed]
- Varela, H.A.; Souza, J.C.M.; Nascimento, R.M.; Araujo, R.F., Jr.; Vasconcelos, R.C.; Cavalcante, R.S.; Guedes, P.M.; Araujo, A.A. Injectable platelet rich fibrin: Cell content, morphological, and protein characterization. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aristizabal, R.F.; Lopez, C.; Alvarez, M.E.; Giraldo, C.; Prades, M.; Carmona, J.U. Long-term cytokine and growth factor release from equine platelet-rich fibrin clots obtained with two different centrifugation protocols. Cytokine 2017, 97, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Major, B.; Vácz, G.; Kuten, O.; Hornyák, I.; Hinsenkamp, A.; Kardos, D.; Bagó, M.; Cseh, D.; Sárközi, A.; et al. The Effects of Hyperacute Serum on the Elements of the Human Subchondral Bone Marrow Niche. Stem Cells Int. 2018, 2018, 4854619. [Google Scholar] [CrossRef] [PubMed]
- Kuten, O.; Simon, M.; Hornyak, I.; De Luna-Preitschopf, A.; Nehrer, S.; Lacza, Z. The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng. Part A 2018, 24, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Di Liddo, R.; Bertalot, T.; Borean, A.; Pirola, I.; Argentoni, A.; Schrenk, S.; Cenzi, C.; Capelli, S.; Conconi, M.T.; Parnigotto, P.P. Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J. Cell. Mol. Med. 2018, 22, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Aulino, P.; Costa, A.; Chiaravalloti, E.; Perniconi, B.; Adamo, S.; Coletti, D.; Marrelli, M.; Tatullo, M.; Teodori, L. Muscle Extracellular Matrix Scaffold Is a Multipotent Environment. Int. J. Med. Sci. 2015, 12, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perniconi, B.; Coletti, D.; Aulino, P.; Costa, A.; Aprile, P.; Santacroce, L.; Chiaravalloti, E.; Coquelin, L.; Chevallier, N.; Teodori, L.; et al. Muscle acellular scaffold as a biomaterial: Effects on C2C12 cell differentiation and interaction with the murine host environment. Front. Physiol. 2014, 5, 354. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Gentile, S.; Palmieri, F.; Paduano, F.; Tatullo, M. Correlation between Surgeon’s experience, surgery complexity and the alteration of stress related physiological parameters. PLoS ONE 2014, 9, e112444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.V.; Shubhashini, N. Platelet rich fibrin: A new paradigm in periodontal regeneration. Cell Tissue Bank. 2013, 14, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Debnath, K.; Chatterjee, A. Clinical and histological evaluation on application of platelet concentrates on depigmented gingival epithelium. J. Indian Soc. Periodontol. 2018, 22, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pirebas, H.G.; Hendek, M.K.; Kisa, U.; Yalim, M.; Erdemir, E.O. Effect of titanium-prepared platelet-rich fibrin treatment on the angiogenic biomarkers in gingival crevicular fluid in infrabony defects of patients with chronic periodontitis: A randomized controlled clinical trial. Niger. J. Clin. Pract. 2018, 21, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Behavior of Gingival Fibroblasts on Titanium Implant Surfaces in Combination with either Injectable-PRF or PRP. Int. J. Mol. Sci. 2017, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.J.; Yang, M.; Zhang, C.; Xue, R.; Zhang, W.; Qin, H.X. Effects of Choukroun’s platelet-rich fibrin on human gingival fibroblasts proliferation, migration and type I collagen secretion. Zhonghua Kou Qiang Yi Xue Za Zhi 2013, 48, 72–76. [Google Scholar] [PubMed]
- De Almeida Barros Mourao, C.F.; Calasans-Maia, M.D.; de Mello Machado, R.C.; de Brito Resende, R.F.; Alves, G.G. The use of platelet-rich fibrin as a hemostatic material in oral soft tissues. Oral Maxillofac. Surg. 2018, 22, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Esmaeili, S.; Fakhr Tabatabayi, S.; Ellini, M.R.; Nekoofar, M.H.; Dummer, P.M. Second-generation Platelet Concentrate (Platelet-rich Fibrin) as a Scaffold in Regenerative Endodontics: A Case Series. J. Endod. 2017, 43, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kokdere, N.N.; Baykul, T.; Findik, Y. The use of platelet-rich fibrin (PRF) and PRF-mixed particulated autogenous bone graft in the treatment of bone defects: An experimental and histomorphometrical study. Dent. Res. J. 2015, 12, 418–424. [Google Scholar]
- Miron, R.J.; Pikos, M.A. Sinus Augmentation Using Platelet-Rich Fibrin With or without a Bone Graft: What Is the Consensus? Compend. Contin. Educ. Dent. 2018, 39, 355–361. [Google Scholar] [PubMed]
- Wong, C.-C.; Kuo, T.-F.; Yang, T.-L.; Tsuang, Y.-H.; Lin, M.-F.; Chang, C.-H.; Lin, Y.-H.; Chan, W.P. Platelet-Rich Fibrin Facilitates Rabbit Meniscal Repair by Promoting Meniscocytes Proliferation, Migration, and Extracellular Matrix Synthesis. Int. J. Mol. Sci. 2017, 18, 1722. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.B.; Mahindra, U.R.; Kini, Y.K.; Bakshi, M.K. Use of Platelet-Rich Fibrin over Skin Wounds: Modified Secondary Intention Healing. J. Cutan. Aesthet. Surg. 2013, 6, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.; Das, P.; Rana, M.M.; Qureshi, A.Q.; Vaidya, K.C.; Ahmed Raziuddin, S.J. Wound Healing and Bone Regeneration in Postextraction Sockets with and without Platelet-rich Fibrin. Ann. Maxillofac. Surg. 2018, 8, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Crisci, A.; Marotta, G.; Licito, A.; Serra, E.; Benincasa, G.; Crisci, M. Use of Leukocyte Platelet (L-PRF) Rich Fibrin in Diabetic Foot Ulcer with Osteomyelitis (Three Clinical Cases Report). Diseases 2018, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Reed, D.A.; Min, L.; Gopinathan, G.; Li, S.; Dangaria, S.J.; Li, L.; Geng, Y.; Galang, M.T.; Gajendrareddy, P.; et al. Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx2. Int. J. Mol. Sci. 2014, 15, 8509–8525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qi, X.; Luo, X.; Li, D.; Wang, H.; Li, T. Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration. Clin. Implant Dent. Relat. Res. 2017, 19, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, H.; Haddadi, P.; Raoofi, S.; Badiee, P.; Dehghani Nazhvani, A. Does Adding Silver Nanoparticles to Leukocyte- and Platelet-Rich Fibrin Improve Its Properties? BioMed Res. Int. 2018, 2018, 8515829. [Google Scholar] [CrossRef] [PubMed]
- Akbari Saeed, T.; Ahmadi ZeydAbadi, M.; Fatemi, A.; Farsinejad, A. In vitro evaluation of decontamination effects on mechanical properties of fibrin membrane. Med. J. Islam. Repub. Iran 2018, 32, 2. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; et al. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorshidi, H.; Raoofi, S.; Bagheri, R.; Banihashemi, H. Comparison of the Mechanical Properties of Early Leukocyte- and Platelet-Rich Fibrin versus PRGF/Endoret Membranes. Int. J. Dent. 2016, 2016, 1849207. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Vacz, G.; Cselenyak, A.; Weszl, M.; Kiss, L.; Lacza, Z. Albumin-coated bioactive suture for cell transplantation. Surg. Innov. 2013, 20, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Vacz, G.; Szabo, T.; Szigyarto, I.C.; Toro, I.; Vamos, B.; Hornyak, I.; Renner, K.; Klara, T.; Szabo, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Simon, M.; Schwarz, C.M.; Masteling, M.; Vacz, G.; Hornyak, I.; Lacza, Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. Biofactors 2017, 43, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Weszl, M.; Skaliczki, G.; Cselenyak, A.; Kiss, L.; Major, T.; Schandl, K.; Bognar, E.; Stadler, G.; Peterbauer, A.; Csonge, L.; et al. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2012, 30, 489–496. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardos, D.; Hornyák, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Várdai, R.; Kállay-Menyhárd, A.; Pinke, B.; Mészáros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci. 2018, 19, 3433. https://doi.org/10.3390/ijms19113433
Kardos D, Hornyák I, Simon M, Hinsenkamp A, Marschall B, Várdai R, Kállay-Menyhárd A, Pinke B, Mészáros L, Kuten O, et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. International Journal of Molecular Sciences. 2018; 19(11):3433. https://doi.org/10.3390/ijms19113433
Chicago/Turabian StyleKardos, Dorottya, István Hornyák, Melinda Simon, Adél Hinsenkamp, Bence Marschall, Róbert Várdai, Alfréd Kállay-Menyhárd, Balázs Pinke, László Mészáros, Olga Kuten, and et al. 2018. "Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System" International Journal of Molecular Sciences 19, no. 11: 3433. https://doi.org/10.3390/ijms19113433