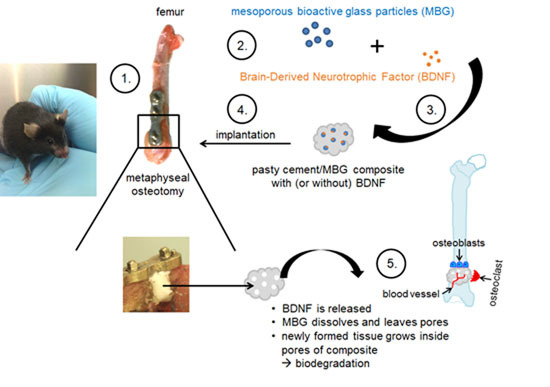
Effects of a Pasty Bone Cement Containing Brain-Derived Neurotrophic Factor-Functionalized Mesoporous Bioactive Glass Particles on Metaphyseal Healing in a New Murine Osteoporotic Fracture Model
Abstract
:1. Introduction
2. Results
2.1. Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR)
2.2. Micro-Computed Tomography (Micro-CT) Analysis
2.3. Histology and Histomorphometry
2.4. Time-Of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS), High-Resolution Scanning Electron Microscopy (HR-SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.5. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.6. CRP Analysis
2.7. Analysis of Total Protein as well as Alkaline Phosphates (ALP) Concentration and Activity in Callus Tissue Cultured In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Bone Substitute Material for Fracture Gap Closure
4.3. Surgical Procedure
4.4. Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR)
4.5. Micro-CT
4.6. Histological Analyses of Sections Embedded in Technovit 9100
4.7. Histomorphometrical Analysis of Fracture Gap and Callus Tissue
4.8. ToF-SIMS, HR-SEM and EDS
4.9. FACS
4.10. CRP Analysis
4.11. ALP Analysis in Callus Tissue Cultured In Vitro
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-TCP | α-tricalcium phosphate |
| ALP | Alkaline phosphatase |
| ASMA | Alpha smooth muscle actin |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMP | Bone morphogenic protein |
| BV | Bone Volume |
| BV/TV | Bone volume fraction |
| CPCs | Calcium phosphate cements |
| CRP | C-reactive protein |
| EDS | Energy-dispersed X-ray spectroscopy |
| HA | Hydroxyapatite |
| HR-SEM | High-resolution scanning electron microscopy |
| KO | Knockout |
| M3 mAChR | M3 muscarinic acetylcholine receptor |
| MBG | Mesoporous bioactive glass |
| Micro-CT | Micro-computed tomography |
| MSCs | Mesenchymal stem cells |
| RT-PCR | Reverse-transcription polymerase chain reaction |
| TGF-β | Transforming growth factor |
| ToF-SIMS | Time-of-Flight Secondary Ion Mass Spectrometry |
| TRAP | Tartrate-resistant acidic phosphatase |
| TV | Total Volume |
| VEGF | Vascular endothelial growth factor |
| WT | Wild type |
References
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Miclau, T.; Chow, S.K.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, S21–S26. [Google Scholar] [CrossRef]
- Doshi, H.K.; Wenxian, P.; Burgula, M.V.; Murphy, D.P. Clinical outcomes of distal femoral fractures in the geriatric population using locking plates with a minimally invasive approach. Geriatr. Orthop. Surg. Rehabil. 2013, 4, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Govindarajan, P.; Schlewitz, G.; Hemdan, N.Y.; Schliefke, N.; Alt, V.; Thormann, U.; Lips, K.S.; Wenisch, S.; Langheinrich, A.C.; et al. Induction of osteoporosis with its influence on osteoporotic determinants and their interrelationships in rats by dexa. Med. Sci. Monit. 2012, 18, BR199–BR207. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.S.; Kneffel, M.; Willscheid, F.; Mathies, F.M.; Kampschulte, M.; Hartmann, S.; Panzer, I.; Durselen, L.; Heiss, C.; Kauschke, V. Altered ultrastructure, density and cathepsin k expression in bone of female muscarinic acetylcholine receptor m3 knockout mice. Int. Immunopharmacol. 2015, 29, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, L.; Chen, W.; Liu, X.; Weir, M.D.; Xu, H.H. Stem cells and calcium phosphate cement scaffolds for bone regeneration. J. Dent. Res. 2014, 93, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meng, H.; Wang, X.; Zhao, C.; Peng, J.; Wang, Y. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med. Sci. Monit. 2016, 22, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A. review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Kilian, O.; Hartmann, S.; Dongowski, N.; Karnati, S.; Baumgart-Vogt, E.; Hartel, F.V.; Noll, T.; Schnettler, R.; Lips, K.S. Bdnf and its trkb receptor in human fracture healing. Ann. Anat. 2014, 196, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Ida-Yonemochi, H.; Yamada, Y.; Yoshikawa, H.; Seo, K. Locally produced bdnf promotes sclerotic change in alveolar bone after nerve injury. PLoS ONE 2017, 12, e0169201. [Google Scholar] [CrossRef] [PubMed]
- Henss, A.; Rohnke, M.; El Khassawna, T.; Govindarajan, P.; Schlewitz, G.; Heiss, C.; Janek, J. Applicability of tof-sims for monitoring compositional changes in bone in a long-term animal model. J. R. Soc. Interface 2013, 10, 20130332. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Veeser, A.; Gockelmann, M.; Simon, U.; Ignatius, A. A novel model to study metaphyseal bone healing under defined biomechanical conditions. Arch. Orthop. Traum. Surg. 2009, 129, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Thormann, U.; El Khawassna, T.; Ray, S.; Duerselen, L.; Kampschulte, M.; Lips, K.; von Dewitz, H.; Heinemann, S.; Heiss, C.; Szalay, G.; et al. Differences of bone healing in metaphyseal defect fractures between osteoporotic and physiological bone in rats. Injury 2014, 45, 487–493. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Zhang, G.; Pan, X.H.; Liu, Z.; Zheng, L.Z.; Chan, C.W.; Lee, K.M.; Cao, Y.P.; Li, G.; Wei, L.; et al. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect model. Bone 2011, 48, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Warriner, A.H.; Patkar, N.M.; Curtis, J.R.; Delzell, E.; Gary, L.; Kilgore, M.; Saag, K. Which fractures are most attributable to osteoporosis? J. Clin. Epidemiol. 2011, 64, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Aghvami, M.; Brunski, J.; Helms, J. Biophysical regulation of osteotomy healing: An animal study. Clin. Implant Dent. Relat. Res. 2017, 19, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Mouraret, S.; Houschyar, K.S.; Hunter, D.J.; Smith, A.A.; Jew, O.S.; Girod, S.; Helms, J.A. Cell viability after osteotomy and bone harvesting: Comparison of piezoelectric surgery and conventional bur. Int. J. Oral Maxillofac. Surg. 2014, 43, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Guo, H.L.; Ding, D.F.; Wu, Y.Y.; Zhao, Y.F.; Gu, X.F.; Zheng, Y.X. Changes of mesenchymal stromal cells mobilization and bone turnover in an experimental bone fracture model in ovariectomized mice. Int. J. Clin. Exp. Pathol. 2015, 8, 10228–10238. [Google Scholar] [PubMed]
- Lill, C.A.; Hesseln, J.; Schlegel, U.; Eckhardt, C.; Goldhahn, J.; Schneider, E. Biomechanical evaluation of healing in a non-critical defect in a large animal model of osteoporosis. J. Orthop. Res. 2003, 21, 836–842. [Google Scholar] [CrossRef]
- Histing, T.; Stenger, D.; Kuntz, S.; Scheuer, C.; Tami, A.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Increased osteoblast and osteoclast activity in female senescence-accelerated, osteoporotic samp6 mice during fracture healing. J. Surg. Res. 2012, 175, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.S.; Efstathopoulos, N.; Kontakis, G.; Kanakaris, N.K.; Giannoudis, P.V. The influence of osteoporosis in femoral fracture healing time. Injury 2009, 40, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kyllonen, L.; D′Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015, 11, 412–434. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, S.; Stahl, J.P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. A 2003, 67, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Y.; de Bruijn, J.D.; de Groot, K.; Zhang, X. Tissue responses of calcium phosphate cement: A. study in dogs. Biomaterials 2000, 21, 1283–1290. [Google Scholar] [CrossRef]
- Schumacher, M.; Lode, A.; Lips, K.; Gelinsky, M. Mesoporous bioactive glass/cap bone cement composites for protein delivery. Tissue Eng. Part A 2015, 21, S372–S373. [Google Scholar]
- Yamamoto, H.; Gurney, M.E. Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 1990, 10, 3469–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnouf, T.; Kuo, Y.P.; Blum, D.; Burnouf, S.; Su, C.Y. Human platelet concentrates: A source of solvent/detergent-treated highly enriched brain-derived neurotrophic factor. Transfusion 2012, 52, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tu, Q.; Bonewald, L.F.; He, X.; Stein, G.; Lian, J.; Chen, J. Effects of mir-335-5p in modulating osteogenic differentiation by specifically downregulating wnt antagonist dkk1. J. Bone Miner. Res. 2011, 26, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, Y.; Sun, Z.; Wang, H.; Zhou, H.; Zhang, L.; Zhang, S.; Cao, X. Mirna-132-3p inhibits osteoblast differentiation by targeting ep300 in simulated microgravity. Sci. Rep. 2015, 5, 18655. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Chen, X.; Yu, X. Micrornas in osteoclastogenesis and function: Potential therapeutic targets for osteoporosis. Int. J. Mol. Sci. 2016, 17, 349. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Guo, Y.; Qi, H.; Fan, K.; Wang, S.; Zhao, H.; Fan, Y.; Xie, J.; Guo, F.; Hou, Y.; et al. M3 subtype of muscarinic acetylcholine receptor promotes cardioprotection via the suppression of mir-376b-5p. PLoS ONE 2012, 7, e32571. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, H.; Xu, C.; Liu, Y.; Wang, H.; Han, H.; Wang, Z. Choline produces cytoprotective effects against ischemic myocardial injuries: Evidence for the role of cardiac m3 subtype muscarinic acetylcholine receptors. Cell. Physiol. Biochem. 2005, 16, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Oury, F.; Yadav, V.K.; Wess, J.; Liu, X.S.; Guo, X.E.; Murshed, M.; Karsenty, G. Signaling through the m(3) muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell Metab. 2010, 11, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kauschke, V.; Gebert, A.; Calin, M.; Eckert, J.; Scheich, S.; Heiss, C.; Lips, K.S. Effects of new beta-type ti-40nb implant materials, brain-derived neurotrophic factor, acetylcholine and nicotine on human mesenchymal stem cells of osteoporotic and non osteoporotic donors. PLoS ONE 2018, 13, e0193468. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lian, D.; Wu, J.; Liu, Y.; Zhu, M.; Sun, J.; He, D.; Li, L. Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental streptococcus pneumoniae meningitis. J. Neuroinflamm. 2017, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Szypula, J.; Cabak, A.; Kiljanski, M.; Boguszewski, D.; Tomaszewski, W. Comparison of biocompatibility of cemented vs. Cementless hip joint endoprostheses based on postoperative evaluation of proinflammatory cytokine levels. Med. Sci. Monit. 2016, 22, 4830–4835. [Google Scholar] [CrossRef] [PubMed]
- Kiran, D.N.; Desai, R. Estimation of c-reactive protein associated with mandibular fracture. J. Maxillofac. Oral Surg. 2012, 11, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B. Clinical utility of biochemical markers of bone remodeling. Clin. Chem. 1999, 45, 1359–1368. [Google Scholar] [PubMed]
- Heinemann, S.; Rossler, S.; Lemm, M.; Ruhnow, M.; Nies, B. Properties of injectable ready-to-use calcium phosphate cement based on water-immiscible liquid. Acta Biomater. 2013, 9, 6199–6207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, C.; Ramaswamy, Y.; Kockrick, E.; Simon, P.; Kaskel, S.; Zreiqat, H. Preparation, characterization and in vitro bioactivity of mesoporous bioactive glasses (mbgs) scaffolds for bone tissue engineering. Microporous Mesoporous Mater. 2008, 112, 494–503. [Google Scholar] [CrossRef]
- Histing, T.; Klein, M.; Stieger, A.; Stenger, D.; Steck, R.; Matthys, R.; Holstein, J.H.; Garcia, P.; Pohlemann, T.; Menger, M.D. A new model to analyze metaphyseal bone healing in mice. J. Surg. Res. 2012, 178, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Kauschke, V.; Kneffel, M.; Floel, W.; Hartmann, S.; Kampschulte, M.; Durselen, L.; Ignatius, A.; Schnettler, R.; Heiss, C.; Lips, K.S. Bone status of acetylcholinesterase-knockout mice. Int. Immunopharmacol. 2015, 29, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Olah, A.J.; Simon, A.; Gaudy, M.; Herrmann, W.; Schenk, R.K. Differential staining of calcified tissues in plastic embedded microtome sections by a modification of movat′s pentachrome stain. Stain Technol. 1977, 52, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.; Dittmann, N.; Schutz, I.; Klein, J.; Lips, K.S. Effect of m3 muscarinic acetylcholine receptor deficiency on collagen antibody-induced arthritis. Arthritis Res. Ther. 2016, 18, 17. [Google Scholar] [CrossRef] [PubMed]









| Primer | Sequence | Length (bp) | Accession No. |
|---|---|---|---|
| Alp for rev | TCAGCTAATGCACAATATCAAGG TCCACATCAGTTCTGTTCTTCG | 87 | NM_007431.2 |
| Ctsk for rev | GAGGCGGCTATATGACCACT CTTTGCCGTGGCGTTATACA | 119 | NM_007802.3 |
| Cx43 for rev | TGCTTCCTCTCACGTCCCAC CGCGATCCTTAACGCCCTTG | 127 | NM_010288.3 |
| ß-actin for rev | TGTTACCAACTGGGACGACA GGGGTGTTGAAGGTCTCAAA | 165 | NM_007393.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauschke, V.; Schneider, M.; Jauch, A.; Schumacher, M.; Kampschulte, M.; Rohnke, M.; Henss, A.; Bamberg, C.; Trinkaus, K.; Gelinsky, M.; et al. Effects of a Pasty Bone Cement Containing Brain-Derived Neurotrophic Factor-Functionalized Mesoporous Bioactive Glass Particles on Metaphyseal Healing in a New Murine Osteoporotic Fracture Model. Int. J. Mol. Sci. 2018, 19, 3531. https://doi.org/10.3390/ijms19113531
Kauschke V, Schneider M, Jauch A, Schumacher M, Kampschulte M, Rohnke M, Henss A, Bamberg C, Trinkaus K, Gelinsky M, et al. Effects of a Pasty Bone Cement Containing Brain-Derived Neurotrophic Factor-Functionalized Mesoporous Bioactive Glass Particles on Metaphyseal Healing in a New Murine Osteoporotic Fracture Model. International Journal of Molecular Sciences. 2018; 19(11):3531. https://doi.org/10.3390/ijms19113531
Chicago/Turabian StyleKauschke, Vivien, Maike Schneider, Annika Jauch, Matthias Schumacher, Marian Kampschulte, Marcus Rohnke, Anja Henss, Coralie Bamberg, Katja Trinkaus, Michael Gelinsky, and et al. 2018. "Effects of a Pasty Bone Cement Containing Brain-Derived Neurotrophic Factor-Functionalized Mesoporous Bioactive Glass Particles on Metaphyseal Healing in a New Murine Osteoporotic Fracture Model" International Journal of Molecular Sciences 19, no. 11: 3531. https://doi.org/10.3390/ijms19113531