Characterization of Heterotrimeric G Protein γ4 Subunit in Rice
Abstract
:1. Introduction
2. Results
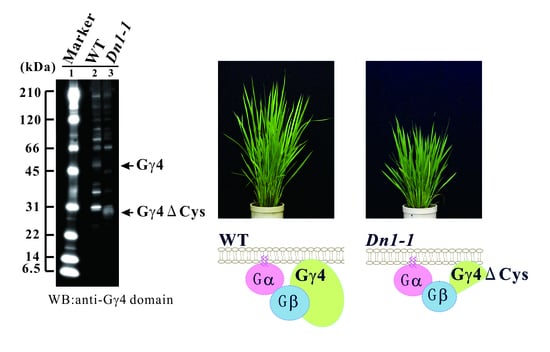
2.1. Rice Heterotrimeric G protein γ4 Gene (RGG4/DEP1/DN1/qPE9-1/OsGGC3) Mutant
2.2. Genomic Structure of RGG4 and Protein Structure of Gγ4
2.3. Gγ4 Candidates Localized in the Plasma Membrane Fraction
2.4. Immunoprecipitation of Gγ4, and Gγ4∆Cys Using Anti-Gγ4 Domain Antibody
2.5. LC-MS/MS Analysis
2.6. Enrichment of Gγ4 and Gγ4∆Cys in the Plasma Membrane Fraction
2.7. Tissue-Specific Accumulation of Gγ4
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Sequencing and Confirmation of RGG4
4.3. RNA Isolation, Reverse Transcription, and cDNA Encoding of the Heterotrimeric G Protein γ4 Subunit
4.4. Preparation of cMS and Plasma Membrane Fractions of Rice
4.5. SDS-PAGE
4.6. Preparation of Trx-Gγ4 and GST-Gγ4 Domain Proteins
4.7. Western Blotting (WB)
4.8. Immunoprecipitation (IP)
4.9. Protein Reduction, Alkylation, and Trypsin Digestion for LC-MS/MS Analysis
4.10. Protein Identification Using Nano LC-MS/MS
4.11. MS Data Analysis
4.12. Gene ID
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| agb1 | Mutant of heterotrimeric G protein β subunit gene in Arabidopsis |
| agg1 | Mutant of heterotrimeric G protein γ1 subunit gene in Arabidopsis |
| agg2 | Mutant of heterotrimeric G protein γ2 subunit gene in Arabidopsis |
| agg3 | Mutant of heterotrimeric G protein γ3 subunit gene in Arabidopsis |
| AGB1 | Heterotrimeric G protein β subunit gene in Arabidopsis |
| AGG1 | Heterotrimeric G protein γ1 subunit gene in Arabidopsis |
| AGG2 | Heterotrimeric G protein γ2 subunit gene in Arabidopsis |
| AGG3 | Heterotrimeric G protein γ3 subunit gene in Arabidopsis |
| d1 | Mutant of heterotrimeric G protein α subunit gene in rice |
| DEP1 | DENCE AND ERRECT PANICLES 1 |
| DN1 | DENCE PANICLE 1 |
| gpa1 | Mutant of heterotrimeric G protein α subunit gene in Arabidopsis |
| Gα | Heterotrimeric G protein α subunit |
| Gβ | Heterotrimeric G protein β subunit |
| Gγ | Heterotrimeric G protein γ subunit |
| GPA1 | Heterotrimeric G protein α subunit gene in Arabidopsis |
| GS3 | GRAIN SIZE 3 gene |
| IP | Immunoprecipitation |
| OsGGC1 | A gene of heterotrimeric G protein γ subunit Type-C in rice, which corresponds to GS3/RGG3 |
| OsGGC2 | A gene of heterotrimeric G protein γ subunit Type-C in rice, which corresponds to RGG5 |
| OsGGC3 | A gene of heterotrimeric G protein γ subunit Type-C in rice, which corresponds to which corresponds to DEP1/RGG4 |
| PM | Plasma membrane |
| qPE9-1 | A quantitative trait locus regulating plant architecture including panicle erectness in rice |
| RGA1 | Heterotrimeric G protein α subunit gene in rice |
| RGB1 | Heterotrimeric G protein β subunit gene in rice |
| RGG1 | Heterotrimeric G protein γ1 subunit gene in rice |
| RGG2 | Heterotrimeric G protein γ2 subunit gene in rice |
| RGG3 | Heterotrimeric G protein γ3 subunit gene in rice |
| RGG4 | Heterotrimeric G protein γ4 subunit gene in rice |
| RGG5 | Heterotrimeric G protein γ5 subunit gene in rice |
| WB | Western blot |
| WT | Wild-type |
| XLG | A gene coding extra-large GTP-binding protein |
| XLG | Extra-large GTP-binding protein |
| xlg | Mutant of a gene coding extra-large GTP-binding protein |
References
- Offermanns, S. Mammalian G-protein function in vivo: New insights through altered gene expression. Rev. Physiol. Biochem. Pharmacol. 2000, 140, 63–133. [Google Scholar] [PubMed]
- Signal Transduction; Gomperts, B.D.; Kramer, I.J.M.; Tatham, P.E.R. (Eds.) Elsevier Inc.: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.; Kostenis, E. Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 2006, 147, S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Temple, B.R.S.; Jones, A.M. The Plant Heterotrimeric G-Protein Complex. Annu. Rev. Plant Biol. 2007, 58, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Urano, D.; Chen, J.-G.; Botella, J.R.; Jones, A.M. Heterotrimeric G protein signalling in the plant kingdom. Open Biol. 2013, 3, 120–186. [Google Scholar] [CrossRef] [PubMed]
- Urano, D.; Miura, K.; Wu, Q.; Iwasaki, Y.; Jackson, D.; Jones, A.M. Plant morphology of heterotrimeric G protein mutants. Plant Cell Physiol. 2016, 57, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.J.; Assmann, S.M. Arabidopsis thaliana ‘extra-large GTP-binding protein’ (AtXLG1): A new class of G-protein. Plant Mol. Biol. 1999, 40, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Assmann, S.M. Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell 2002, 14, S355–S373. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yanofsky, M.F.; Meyerowitz, E.M. Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1990, 87, 3821–3825. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.A.; Garnaat, C.W.; Mukai, K.; Hu, Y.; Ma, H. Isolation of cDNAs encoding guanine nucleotide-binding protein β-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc. Natl. Acad. Sci. USA 1994, 91, 9554–9558. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Botella, J.R. Completing the heterotrimer: Isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc. Natl. Acad. Sci. USA 2000, 97, 14784–14788. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Botella, J.R. Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim. Biophys. Acta 2001, 1520, 147–153. [Google Scholar] [CrossRef]
- Chakravorty, D.; Trusov, Y.; Zhang, W.; Acharya, B.R.; Sheahan, M.B.; McCurdy, D.W.; Assmann, S.M.; Botella, J.R. An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J. 2011, 67, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Regan, M.; Furukawa, H.; Jackson, D. Role of heterotrimeric Gα proteins in maize development and enhancement of agronomic traits. PLoS Genet. 2018, 14, e1007374. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Tsubouchi, H.; Iwasaki, Y.; Asahi, T. Molecular cloning and characterization of a cDNA for the α subunit of a G protein from rice. Plant Cell Physiol. 1995, 36, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Iwasaki, Y.; Asahi, T. Molecular cloning and characterization of a cDNA for the β subunit of a G protein from rice. Plant Cell Physiol. 1996, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.; Mizutani, T.; Tamaki, H.; Kumagai, H.; Kamiya, T.; Hirobe, A.; Fujisawa, Y.; Kato, H.; Iwasaki, Y. Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J. 2004, 38, 320–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qian, Q.; Liu, Z.; Sun, H.; He, S.; Luo, D.; Xia, G.; Chu, C.; Li, J.; Fu, X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 2009, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Taguchi-Shiobara, F.; Kawagoe, Y.; Kato, H.; Onodera, H.; Tagiri, A.; Hara, N.; Miyao, A.; Hirochika, H.; Kitano, H.; Yano, M.; et al. A loss-of-function mutation of rice DENSE PANICLE 1 causes semi-dwarfness and slightly increased number of spikelets. Breed. Sci. 2011, 61, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Botella, J.R. Can heterotrimeric G proteins help to feed the world? Trend Plant Sci. 2012, 17, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, J.; Li, Z.; Yi, C.; Liu, J.; Zhang, H.; Tang, S.; Gu, M.; Liang, G. Deletion in a Quantitative Trait Gene qPE9-1 Associated with Panicle Erectness Improves Plant Architecture During Rice Domestication. Genetics 2009, 183, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Trusov, Y.; Chakravorty, D.; Botella, J.R. Diversity of heterotrimeric G-protein γ subunits in plants. BMC Res. Notes 2012, 5, 608. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Pandey, S.; Assmann, S.M. Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J. 2008, 53, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Chen, J.-G.; Young, J.C.; Im, K.-H.; Sussman, M.R.; Jones, A.M. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 2001, 292, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Lease, K.A.; Wen, J.; Li, J.; Doke, J.T.; Liscum, E.; Walker, J.C. A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell 2001, 13, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Chen, J.-G.; Temple, B.; Boyes, D.C.; Alonso, J.M.; Davis, K.R.; Ecker, J.R.; Jones, A.M. The β-subunit of Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 2003, 15, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Trusov, Y.; Rookes, J.E.; Tilbrook, K.; Chakravorty, D.; Mason, M.G.; Anderson, D.; Chen, J.-G.; Jones, A.M.; Botella, J.R. Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell 2007, 19, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Kato, T.; Ohki, S.; Ishikawa, A.; Kitano, H.; Sasaki, T.; Asahi, T.; Iwasaki, Y. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci. USA 1999, 96, 7575–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashikari, M.; Wu, J.; Yano, M.; Sasaki, T.; Yoshimura, A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 1999, 96, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Sun, S.; Yao, J.; Wang, C.; Yu, S.; Xu, C.; Li, X.; Zhang, Q. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 19579–19584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izawa, Y.; Takayanagi, Y.; Inaba, N.; Abe, Y.; Minami, M.; Fujisawa, Y.; Kato, H.; Ohki, S.; Kitano, H.; Iwasaki, Y. Function and expression pattern of the α subunit of the heterotrimeric G protein in rice. Plant Cell Physiol. 2010, 51, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Utsunimiya, U.; Samejima, C.; Takayanagi, Y.; Izawa, Y.; Yoshida, T.; Sawada, Y.; Fijisawa, Y.; Kato, H.; Iwasaki, Y. Suppression of the rice heterotrimeric G protein β-subunit gene, RGB1, causes dwarfism and browning of internodes and lamina joint regions. Plant J. 2011, 67, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Takano-Kai, N.; Jiang, H.; Powell, A.; McCouch, S.; Takamure, I.; Furuya, N.; Doi, K.; Yoshimura, A. Multiple and independent origins of short seeded alleles of GS3 in rice. Breed. Sci. 2013, 63, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Ullah, H.; Jones, A.M.; Assmann, S.M. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 2001, 292, 2070–2072. [Google Scholar] [CrossRef] [PubMed]
- Coursol, S.; Fan, L.M.; Le Stunff, H.; Spiegel, S.; Gilroy, S.; Assmann, S.M. Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 2003, 423, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Lapik, Y.R.; Kaufman, L.S. The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein α-subunit GPA1 and regulates seed germination and early seedling development. Plant Cell 2003, 15, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Assmann, S.M. The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell 2004, 16, 1616–1632. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Zhang, W.; Deng, F.; Zhao, J.; Wang, X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 2006, 312, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi-Tanaka, M.; Fujisawa, Y.; Kobayashi, M.; Ashikari, M.; Iwasaki, Y.; Kitano, H.; Matsuoka, M. Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 2000, 97, 11638–11643. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Chen, J.G.; Wang, S.; Jones, A.M. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 2002, 129, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Pandey, S.; Huang, J.; Alonso, J.M.; Ecker, J.R.; Assmann, S.M.; Jones, A.M. GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol. 2004, 135, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Hwang, Y.S.; Zhu, T.; Jones, R.L. Global patterns of gene expression in the aleurone of wild-type and dwarf1 mutant rice. Plant Physiol. 2006, 140, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-G.; Jones, A.M. AtRGS1 function in Arabidopsis thaliana. Methods Enzymol. 2004, 389, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Taylor, J.P.; Chen, J.G.; Uhrig, J.F.; Schnell, D.J.; Nakagawa, T.; Korth, K.L.; Jones, A.M. The plastid protein THYLAKOID FORMATION1 and the plasma membrane G-protein GPA1 interact in a novel sugar-signaling mechanism in Arabidopsis. Plant Cell 2006, 18, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Warpeha, K.M.; Hamm, H.E.; Rasenick, M.M.; Kaufman, L.S. A blue-light-activated GTP-binding protein in the plasma membranes of etiolated peas. Proc. Natl. Acad. Sci. USA 1991, 88, 8925–8929. [Google Scholar] [CrossRef] [PubMed]
- Warpeha, K.M.; Lateef, S.S.; Lapik, Y.; Anderson, M.; Lee, B.S.; Kaufman, L.S. G-Protein-Coupled Receptor 1, G-Protein Gα-Subunit 1, and Prephenate Dehydratase 1 Are Required for Blue Light-Induced Production of Phenylalanine in Etiolated Arabidopsis. Plant Physiol. 2006, 140, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Wang, S.; Chen, J.G.; Jones, A.M.; Fedoroff, N.V. Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 2005, 17, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Suharsono, U.; Fujisawa, Y.; Kawasaki, T.; Iwasaki, Y.; Satoh, H.; Shimamoto, K. The heterotrimeric G protein α subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 2002, 99, 13307–13312. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Umemura, K.; Teraoka, T.; Usami, H.; Fujisawa, Y.; Iwasaki, Y. Role of the α subunit of heterotrimeric G-protein in probenazole-inducing defense signaling in rice. J. Gen. Plant Pathol. 2003, 69, 83–86. [Google Scholar] [CrossRef]
- Komatsu, S.; Yang, G.; Hayashi, N.; Kaku, H.; Umemura, K.; Iwasaki, Y. Alterations by a defect in a rice G protein α subunit in probenazole and pathogen-induced responses. Plant Cell Environ. 2004, 27, 947–957. [Google Scholar] [CrossRef]
- Lieberherr, D.; Thao, N.P.; Nakashima, A.; Umemura, K.; Kawasaki, T.; Shimamoto, K. A sphingolipid licitor-inducible mitogen-activated protein kinase is regulated by the small GTPase OsRac1 and heterotrimeric G-protein in rice. Plant Physiol. 2005, 138, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Klopffleish, K.; Phan, N.; Augstin, K.; Bayne, R.; Booker, K.S.; Bolella, J.; Carpita, N.C.; Carr, T.; Chen, J.-C.; Cooke, T.R.; et al. Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol. Syst. Biol. 2011, 7, 532. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-G.; Willard, F.S.; Huang, J.; Liang, J.; Chasse, S.A.; Jones, A.M.; Siderovski, D.P. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 2003, 301, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Assmann, S.M.; Fedoroff, N.V. Characterization of the Arabidopsis Heterotrimeric G Protein. J. Biol. Chem. 2008, 283, 13913–13922. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, L.; Mao, H.; Shao, L.; Li, X.; Xiao, J.; Ouyang, Y.; Zhang, Q. A G-protein pathway determines grain size in rice. Nat. Commun. 2018, 9, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Takano-Kai, N.; Jiang, H.; Kubo, T.; Sweeney, M.; Matsumoto, T.; Kanamori, H.; Padhukasahasram, B.; Bustamante, C.; Yoshimura, A.; Doi, K.; et al. Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 2009, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qian, Q.; Wu, K.; Lou, J.; Wang, S.; Zhang, C.; Ma, Y.; Lie, Q.; Huang, X.; Yuan, Q.; et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 2014, 46, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Kunihiro, S.; Saito, T.; Matsuda, T.; Inoue, M.; Kuramata, M.; Taguchi-Shibaoka, F.; Youssefian, S.; Berberich, T.; Kusano, T. Rice DEP1, encoding a highly cycteine-rich G protein γ subunit, confers cadmium tolerance on yeast cells and plants. J. Exp. Bot. 2013, 64, 4517–4527. [Google Scholar] [CrossRef] [PubMed]
- Molecular Cloning; Sambrook, J.; Russell, D.W. (Eds.) Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Yoshida, S.; Uemura, M.; Niki, T.; Sakai, A.; Gusta, L.V. Partition of membrane particles in aqueous two-polymer phase system and its partial use for purification of plasma membranes from plants. Plant Physiol. 1983, 72, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kato, T.; Kaidoh, T.; Ishikawa, A.; Asahi, T. Characterization of the putative α subunit of a heterotrimeric G protein in rice. Plant Mol. Biol. 1997, 34, 563–572. [Google Scholar] [CrossRef] [PubMed]






| (A) | |||||
| Fragments | Observed | Mr (Expt) | Mr (Calc) | Expect | Peptide |
| 1 | 706.8601 | 1411.7057 | 1411.7129 | 1.80 × 10−6 | M.GEEAVVMEAPRPK.S |
| 2 | 440.7008 | 879.3871 | 879.3909 | 0.0015 | R.YPDLCGR.R |
| 3 | 608.8365 | 1215.6585 | 1215.6645 | 9.20 × 10−7 | R.MQLEVQILSR.E |
| 4 | 375.7118 | 749.409 | 749.4323 | 0.0075 | R.EITFLK.D |
| 5 | 799.3979 | 1596.7812 | 1596.7896 | 1.20 × 10−6 | K.DELHFLEGAQPVSR.S |
| 6 | 1036.5248 | 1035.5175 | 1035.5237 | 2.10 × 10−5 | K.EINEFVGTK.H |
| 7 | 460.7616 | 919.5086 | 919.5127 | 0.0077 | K.HDPLIPTK.R |
| 8 | 905.3625 | 1808.7105 | 1808.721 | 7.50 × 10−7 | K.LCICISCLCYCCK.C |
| 9 | 904.4171 | 903.4098 | 903.416 | 0.0006 | K.SLYSCFK.I |
| 10 | 751.3766 | 750.3693 | 750.3734 | 0.00025 | K.IPSCFK.S |
| 11 | 856.6377 | 2566.8913 | 2566.909 | 66.70 × 10−8 | K.SQCNCSSPNCCTCTLPSCSCK.G |
| 12 | 710.2448 | 1418.4751 | 1418.4836 | 1.30 × 10−8 | R.CADCFSCSCPR.C |
| 13 | 581.7522 | 1161.4898 | 1161.4947 | 3.90 × 10−7 | R.CSSCFNIFK.C |
| 14 | 725.2517 | 1448.4889 | 1448.4975 | 7.00 × 10−7 | K.CSCAGCCSSLCK.C |
| 15 | 620.7291 | 1239.4437 | 1239.4505 | 1.10 × 10−5 | R.NPCCLSGCLC |
| (B) | |||||
| Fragments | Observed | Mr (expt) | Mr (Calc) | Expect | Peptide |
| 1 | 706.8604 | 1411.7063 | 1411.7129 | 0.000016 | M.GEEAVVMEAPRPK.S |
| 2 | 440.7009 | 879.3872 | 879.3909 | 0.002 | R.YPDLCGR.R |
| 3 | 608.8362 | 1215.6579 | 1215.6645 | 5.6 × 10−7 | R.MQLEVQILSR.E |
| 4 | 375.7219 | 749.4292 | 749.4323 | 0.0019 | R.EITFLK.D |
| 5 | 799.3994 | 1596.7842 | 1596.7896 | 0.000046 | K.DELHFLEGAQPVSR.S |
| 6 | 1036.5227 | 1035.5154 | 1035.5237 | 0.000024 | K.EINEFVGTK.H |
| 7 | 460.7607 | 919.5068 | 919.5127 | 0.018 | K.HDPLIPTK.R |
| 8 | 905.3626 | 1808.7107 | 1808.721 | 0.0000015 | K.LCICISCLCYCCK.C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuta, S.; Nishiyama, A.; Chaya, G.; Itoh, T.; Miura, K.; Iwasaki, Y. Characterization of Heterotrimeric G Protein γ4 Subunit in Rice. Int. J. Mol. Sci. 2018, 19, 3596. https://doi.org/10.3390/ijms19113596
Matsuta S, Nishiyama A, Chaya G, Itoh T, Miura K, Iwasaki Y. Characterization of Heterotrimeric G Protein γ4 Subunit in Rice. International Journal of Molecular Sciences. 2018; 19(11):3596. https://doi.org/10.3390/ijms19113596
Chicago/Turabian StyleMatsuta, Sakura, Aki Nishiyama, Genki Chaya, Takafumi Itoh, Kotaro Miura, and Yukimoto Iwasaki. 2018. "Characterization of Heterotrimeric G Protein γ4 Subunit in Rice" International Journal of Molecular Sciences 19, no. 11: 3596. https://doi.org/10.3390/ijms19113596