Visfatin Promotes Wound Healing through the Activation of ERK1/2 and JNK1/2 Pathway
Abstract
:1. Introduction
2. Results
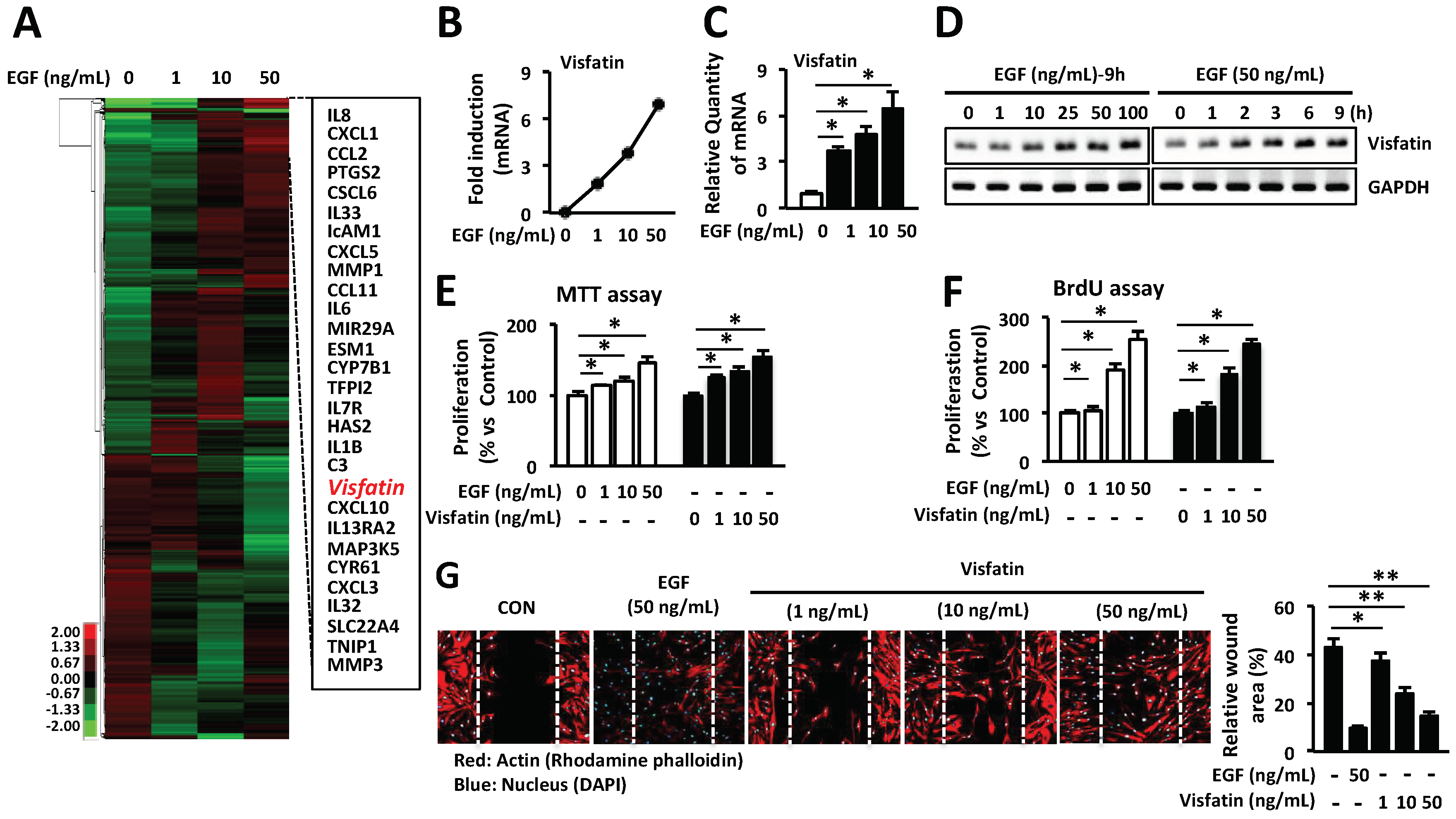
2.1. Visfatin Enhances the Proliferation and Migration of HDFs
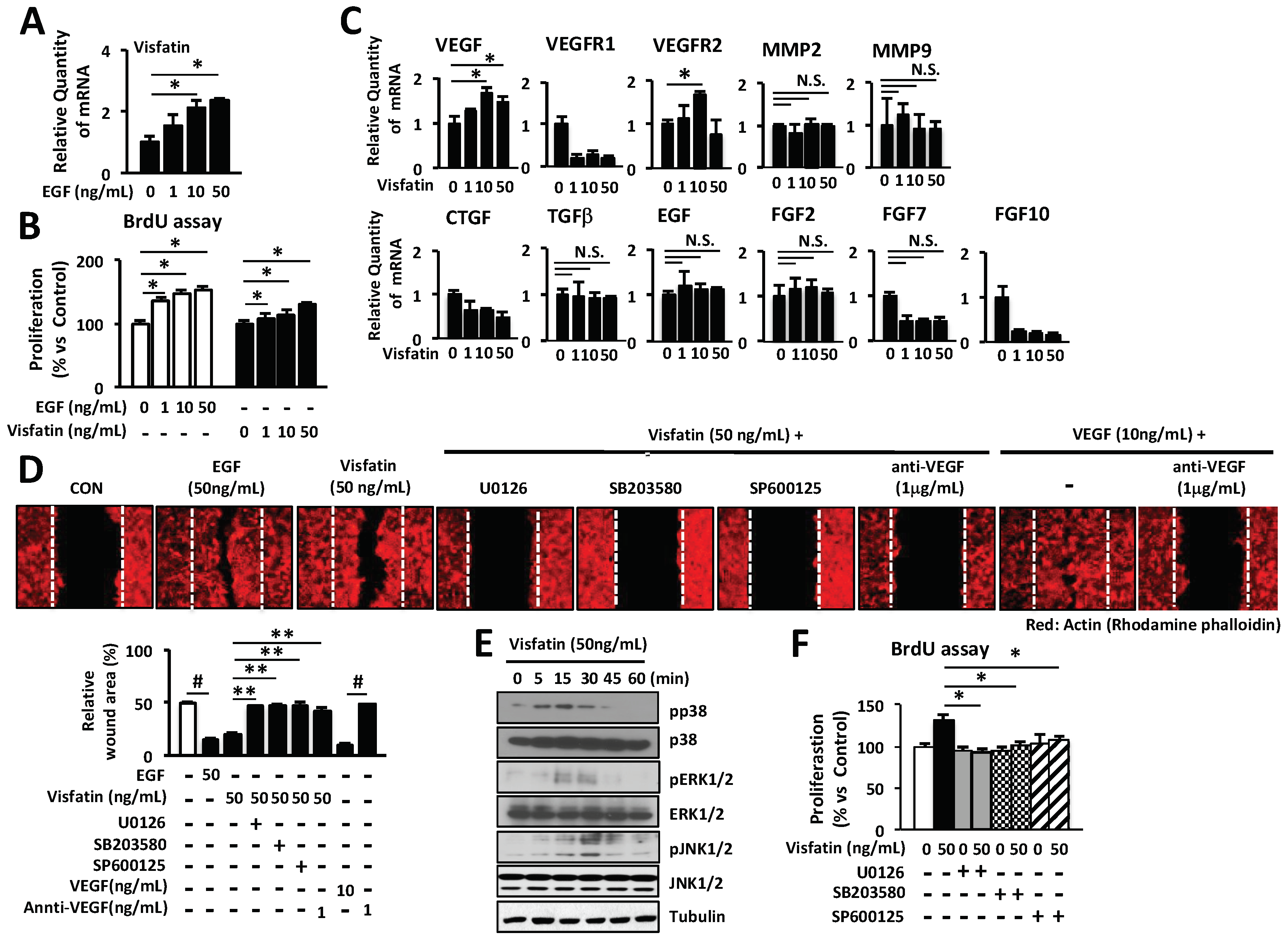
2.2. Involvement of VEGF in Visfatin-Mediated Wound Healing
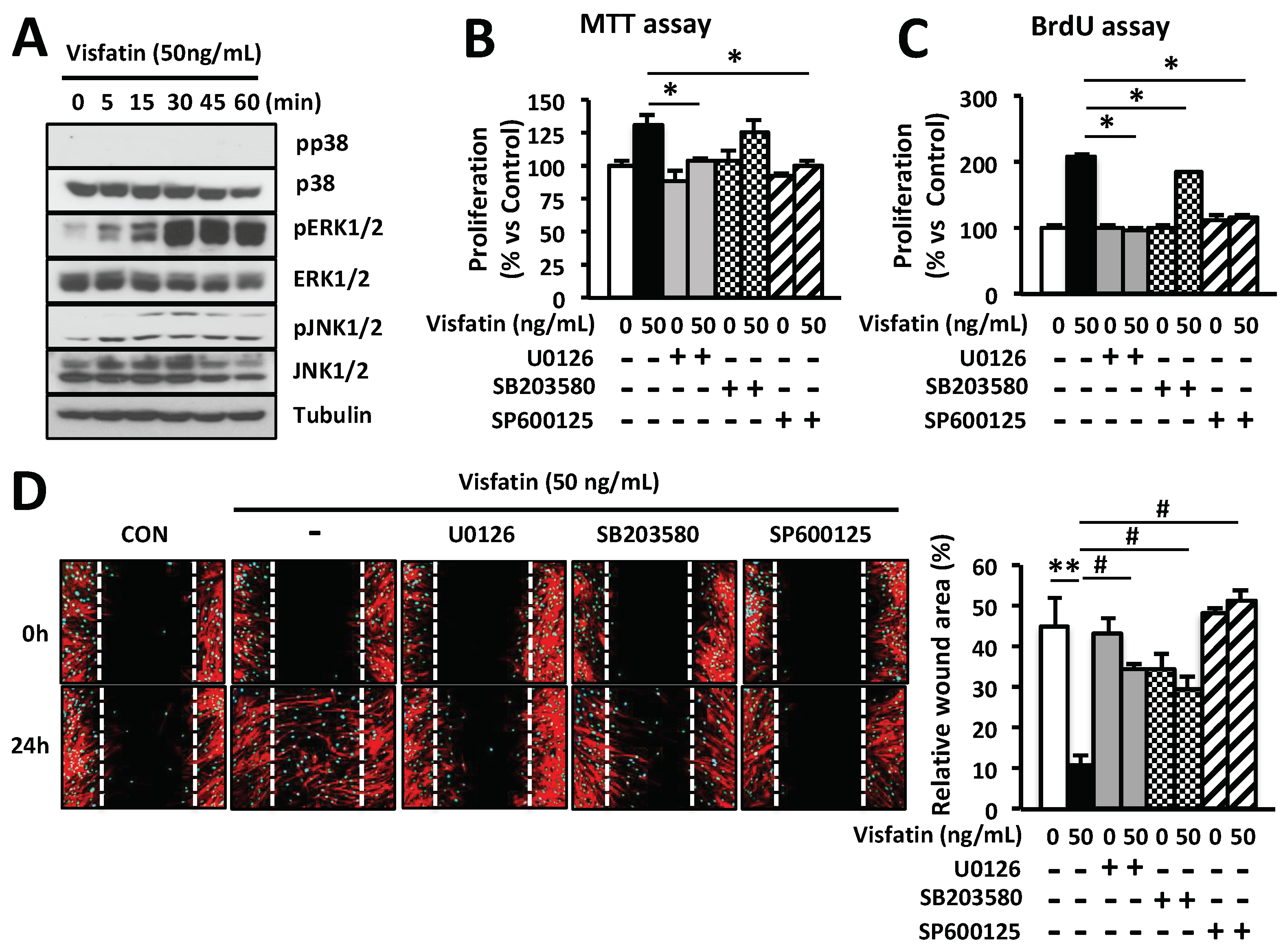
2.3. Wound Healing Is Induced by Visfatin via ERK1/2 and JNK1/2 Activation in HDFs
2.4. Wound Healing Activity Induced by Visfatin in Keratinocytes
2.5. Induction of Wound Healing by Visfatin In Vivo
3. Discussion
4. Materials and Methods
4.1. Mice and Cell Culture
4.2. Reagents and Antibody
4.3. cDNA Microarray
4.4. MTT and BrdU Proliferation Assays In Vitro
4.5. Migration Assay In Vitro
4.6. Wound Healing Assay In Vivo
4.7. Histologic Analysis of Wounds and Immunofluorescence Microscopy
4.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Real-Time Quantitative PCR (Q-PCR) Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.A.; Horsley, V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 2013, 140, 1517–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shook, B.; Xiao, E.; Kumamoto, Y.; Iwasaki, A.; Horsley, V. CD301b+ Macrophages Are Essential for Effective Skin Wound Healing. J. Investig. Dermatol. 2016, 136, 1885–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.B.; Yoon, W.Y.; Han, S.K.; Jeong, S.H.; Cui, Z.J.; Kim, W.K. Cytotoxicity of silver dressings on diabetic fibroblasts. Int. Wound J. 2013, 10, 306–312. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, E.A. Extracellular matrix and keratinocyte migration. Clin. Exp. Dermatol. 2001, 26, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117 Pt 5, 667–675. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Barisic-Dujmovic, T.; Boban, I.; Clark, S.H. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. J. Cell. Physiol. 2010, 222, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Spiekstra, S.W.; Breetveld, M.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Reg. 2007, 15, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; McNiece, I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 1994, 14, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.; Garten, A.; Petzold, S.; Beck-Sickinger, A.G.; Bluher, M.; Stumvoll, M.; Fasshauer, M. Visfatin/PBEF/Nampt: Structure, regulation and potential function of a novel adipokine. Clin. Sci. 2008, 115, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K. Is PBEF/visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? Curr. Hypertens. Rep. 2007, 9, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschen, A.R.; Gerner, R.R.; Tilg, H. Pre-B cell colony enhancing factor/NAMPT/visfatin in inflammation and obesity-related disorders. Curr. Pharm. Des. 2010, 16, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Hug, C.; Lodish, H.F. Visfatin: A new adipokine. Science 2005, 307, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Laudes, M.; Oberhauser, F.; Schulte, D.M.; Freude, S.; Bilkovski, R.; Mauer, J.; Rappl, G.; Abken, H.; Hahn, M.; Schulz, O.; et al. Visfatin/PBEF/Nampt and resistin expressions in circulating blood monocytes are differentially related to obesity and type 2 diabetes in humans. Horm. Metab. Res. 2010, 42, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.B.; Yndestad, A.; Skjelland, M.; Oie, E.; Dahl, A.; Michelsen, A.; Damas, J.K.; Tunheim, S.H.; Ueland, T.; Smith, C.; et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possible role in inflammation and plaque destabilization. Circulation 2007, 115, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.H.; Li, Y.; Parodo, J.; Kapus, A.; Fan, L.; Rotstein, O.D.; Marshall, J.C. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Investig. 2004, 113, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, D.; Majewski, P.; Schulte, L.C.; Korn, J.M.; Young, R.A.; Lander, E.S.; Hacohen, N. The plasticity of dendritic cell responses to pathogens and their components. Science 2001, 294, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Zaidi, M. TNF regulates cellular NAD+ metabolism in primary macrophages. Biochem. Biophys. Res. Commun. 2006, 342, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Kim, E.O.; Jeong, B.R.; Min, Y.J.; Park, J.W.; Kim, E.S.; Namgoong, I.S.; Kim, Y.I.; Lee, B.J. Visfatin stimulates proliferation of MCF-7 human breast cancer cells. Mol. Cells 2010, 30, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Mayhall, K.; Maxwell, N.M.; Kandil, E.; Coppola, D. Nicotinamide phosphoribosyltransferase in malignancy: A review. Genes Cancer 2013, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Bae, S.K.; Choi, K.S.; Park, S.Y.; Jun, H.O.; Lee, J.Y.; Jang, H.O.; Yun, I.; Yoon, K.H.; Kim, Y.J.; et al. Visfatin promotes angiogenesis by activation of extracellular signal-regulated kinase 1/2. Biochem. Biophys. Res. Commun. 2007, 357, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Punn, A.; Chen, J.; Randeva, H.S. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: Novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 2008, 78, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Shukla, P.C.; Quan, A.; Teoh, H.; Szmitko, P.E.; Peterson, M.D.; Gupta, M.; Al-Omran, M.; Verma, S. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: Translational implications for atherosclerosis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1440–E1449. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xiao, Z.J.; Liu, Z.G.; Gong, H.Y.; Yuan, Q.; Wang, S.; Li, Y.J.; Jiang, D.J. Involvement of dimethylarginine dimethylaminohydrolase-2 in visfatin-enhanced angiogenic function of endothelial cells. Diabetes Metab. Res. Rev. 2009, 25, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M. Nicotinamide phosphoribosyl-transferase/visfatin: A missing link between overweight/obesity and postmenopausal breast cancer? Potential preventive and therapeutic perspectives and challenges. Med. Hypotheses 2012, 79, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, T.Y.; Guan, Y.F.; Su, D.F.; Fan, G.R.; Miao, C.Y. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: Role of nicotinamide mononucleotide. Cardiovasc. Res. 2009, 81, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Birkenhauer, E.; Neethirajan, S. A double-edged sword: The role of VEGF in wound repair and chemoattraction of opportunist pathogens. Int. J. Mol. Sci. 2015, 16, 7159–7172. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Zankov, D.P.; Kurokawa-Seo, M.; Ogita, H. Vascular Endothelial Growth Factor-A Exerts Diverse Cellular Effects via Small G Proteins, Rho and Rap. Int. J. Mol. Sci. 2018, 19, 1203. [Google Scholar] [CrossRef] [PubMed]
- Cartland, S.P.; Genner, S.W.; Zahoor, A.; Kavurma, M.M. Comparative Evaluation of TRAIL, FGF-2 and VEGF-A-Induced Angiogenesis In Vitro and In Vivo. Int. J. Mol. Sci. 2016, 17, 2025. [Google Scholar] [CrossRef] [PubMed]
- Han, N.K.; Jeong, Y.J.; Pyun, B.J.; Lee, Y.J.; Kim, S.H.; Lee, H.J. Geranylgeranylacetone Ameliorates Intestinal Radiation Toxicity by Preventing Endothelial Cell Dysfunction. Int. J. Mol. Sci. 2017, 18, 2103. [Google Scholar] [CrossRef] [PubMed]
- Trompezinski, S.; Berthier-Vergnes, O.; Denis, A.; Schmitt, D.; Viac, J. Comparative expression of vascular endothelial growth factor family members, VEGF-B, -C and -D, by normal human keratinocytes and fibroblasts. Exp. Dermatol. 2004, 13, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Stroncek, J.D.; Reichert, W.M. Overview of Wound Healing in Different Tissue Types. In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Nuclear factor-kappaB induction by visfatin in human vascular endothelial cells: Its role in MMP-2/9 production and activation. Diabetes Care 2008, 31, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Bae, M.K.; Kim, S.R.; Lee, J.H.; Wee, H.J.; Bae, S.K. Upregulation of fibroblast growth factor-2 by visfatin that promotes endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2009, 379, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Qiao, S.B.; Yuan, J.S.; Liu, D.Q. Visfatin stimulates production of monocyte chemotactic protein-1 and interleukin-6 in human vein umbilical endothelial cells. Horm. Metab. Res. 2009, 41, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of RAGE, MAPK and NF-kappaB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Li, X.; Yi, C.G.; Pan, H.; He, G.D.; Yu, Q.; Jiang, L.F.; Xu, W.H.; Li, Z.J.; Ding, J.; et al. Angiotensin II activates connective tissue growth factor and induces extracellular matrix changes involving Smad/activation and p38 mitogen-activated protein kinase signalling pathways in human dermal fibroblasts. Exp. Dermatol. 2009, 18, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.M.; Jeong, K.H.; Kim, J.E.; Park, Y.M.; Kang, H. Hair growth-promotion effects of different alternating current parameter settings are mediated by the activation of Wnt/beta-catenin and MAPK pathway. Exp. Dermatol. 2015, 24, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Sun, L.; Zhu, N.; Qi, F. Kindlin-1 contributes to EGF-induced re-epithelialization in skin wound healing. Int. J. Mol. Med. 2017, 39, 949–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rongvaux, A.; Shea, R.J.; Mulks, M.H.; Gigot, D.; Urbain, J.; Leo, O.; Andris, F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 2002, 32, 3225–3234. [Google Scholar] [CrossRef] [Green Version]
- Brentano, F.; Schorr, O.; Ospelt, C.; Stanczyk, J.; Gay, R.E.; Gay, S.; Kyburz, D. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007, 56, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Kanda, N.; Noda, S.; Tatsuta, A.; Kamata, M.; Shibata, S.; Asano, Y.; Sato, S.; Watanabe, S.; Tada, Y. Visfatin enhances the production of cathelicidin antimicrobial peptide, human beta-defensin-2, human beta-defensin-3, and S100A7 in human keratinocytes and their orthologs in murine imiquimod-induced psoriatic skin. Am. J. Pathol. 2013, 182, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Yang, Y.H.; Su, J.H.; Chang, H.L.; Hou, M.F.; Yuan, S.S. High visfatin expression in breast cancer tissue is associated with poor survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Umesh, S.; Thota, B.; Tandon, A.; Pandey, P.; Hegde, A.S.; Balasubramaniam, A.; Chandramouli, B.A.; Santosh, V.; Rao, M.R.; et al. PBEF1/NAmPRTase/Visfatin: A potential malignant astrocytoma/glioblastoma serum marker with prognostic value. Cancer Biol. Ther. 2008, 7, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hufton, S.E.; Moerkerk, P.T.; Brandwijk, R.; de Bruine, A.P.; Arends, J.W.; Hoogenboom, H.R. A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett. 1999, 463, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Van Beijnum, J.R.; Moerkerk, P.T.; Gerbers, A.J.; De Bruine, A.P.; Arends, J.W.; Hoogenboom, H.R.; Hufton, S.E. Target validation for genomics using peptide-specific phage antibodies: A study of five gene products overexpressed in colorectal cancer. Int. J. Cancer 2002, 101, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.T.; Mistry, T.; Brown, J.E.; Digby, J.E.; Adya, R.; Desai, K.M.; Randeva, H.S. A novel role for the adipokine visfatin/pre-B cell colony-enhancing factor 1 in prostate carcinogenesis. Peptides 2010, 31, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Van der Veer, E.; Nong, Z.; O’Neil, C.; Urquhart, B.; Freeman, D.; Pickering, J.G. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ. Res. 2005, 97, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Asai, J.; Takenaka, H.; Hirakawa, S.; Sakabe, J.; Hagura, A.; Kishimoto, S.; Maruyama, K.; Kajiya, K.; Kinoshita, S.; Tokura, Y.; et al. Topical simvastatin accelerates wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis. Am. J. Pathol. 2012, 181, 2217–2224. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| hCOL1A1 | CATGACCGAGACGTGTGGAA | GGCAGTTCTTGGTCTCGTCA |
| hCOL3A1 | TGTTCCAGGAGCTAAAGGCG | CTCCTGGGATGCCATTTGGT |
| hCOL4A1 | TTTTGTGATGCACACCAGCG | AGTAATTGCAGGTCCCACGG |
| hCOL5A1 | AGACATGGGCATCAAGGGTG | CCGAGTTTTCCCTTCTCCCC |
| hMMP2 | CCCTGTGTCTTCCCCTTCAC | GTAGTTGGCTGTGGTCG |
| hMMP9 | GGACAAGCTCTTCGGCTTCT | TCGCTGGTACAGGTCGAGTA |
| hFGF2 | AAAAACGGGGGCTTCTTCCT | AGCCAGGTAACGGTTAGCAC |
| hFGF7 | TGGATCCTGCCAACTTTGCT | TTCTTGTGTGTCGCTCAGGG |
| hFGF10 | GGAGCTACAATCACCT | ACGGGCAGTTCTCCTTCTTG |
| hEGF | AGTCCGTGACTTGCAAGAGG | CCTCTTCTTCCCTAGCCCCT |
| hTGFβ | TGGTGGAAACCCACAACGAA | GAGCAACACGGGTTCAGGTA |
| hCTGF | GTTTGGCCCAGACCCAACTA | GGCTCTGCTTCTCTAGCCTG |
| hVEGF | CTTGCCTTGCTGCTCTACCT | GCAGTAGCTGCGCTGATAGA |
| hVisfatin | TCGGTTCTGGTGGAGGTTTG | TTGGGATCAGCAACTGGGTC |
| hVEGFR1 | GGGGGAAGCAGCCCATAAAT | GCCAGTGTGGTTTGCTTGAG |
| hVEGFR2 | CGGTCAACAAAGTCGGGAGA | CAGTGCACCACAAAGACACG |
| hGAPDH | TCCTGCACCACCAACTGTT | GTCCACTGTCTTCTGGGTGG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-C.; Song, J.; Lee, A.; Cho, D.; Kim, T.S. Visfatin Promotes Wound Healing through the Activation of ERK1/2 and JNK1/2 Pathway. Int. J. Mol. Sci. 2018, 19, 3642. https://doi.org/10.3390/ijms19113642
Lee B-C, Song J, Lee A, Cho D, Kim TS. Visfatin Promotes Wound Healing through the Activation of ERK1/2 and JNK1/2 Pathway. International Journal of Molecular Sciences. 2018; 19(11):3642. https://doi.org/10.3390/ijms19113642
Chicago/Turabian StyleLee, Byung-Cheol, Jisun Song, Arim Lee, Daeho Cho, and Tae Sung Kim. 2018. "Visfatin Promotes Wound Healing through the Activation of ERK1/2 and JNK1/2 Pathway" International Journal of Molecular Sciences 19, no. 11: 3642. https://doi.org/10.3390/ijms19113642





