In-and-Out Molecular Changes Linked to the Type 2 Diabetes Remission after Bariatric Surgery: An Influence of Gut Microbes on Mitochondria Metabolism
Abstract
:1. Introduction
2. Results
2.1. Bioclinical Outcome
2.2. Metabolomics
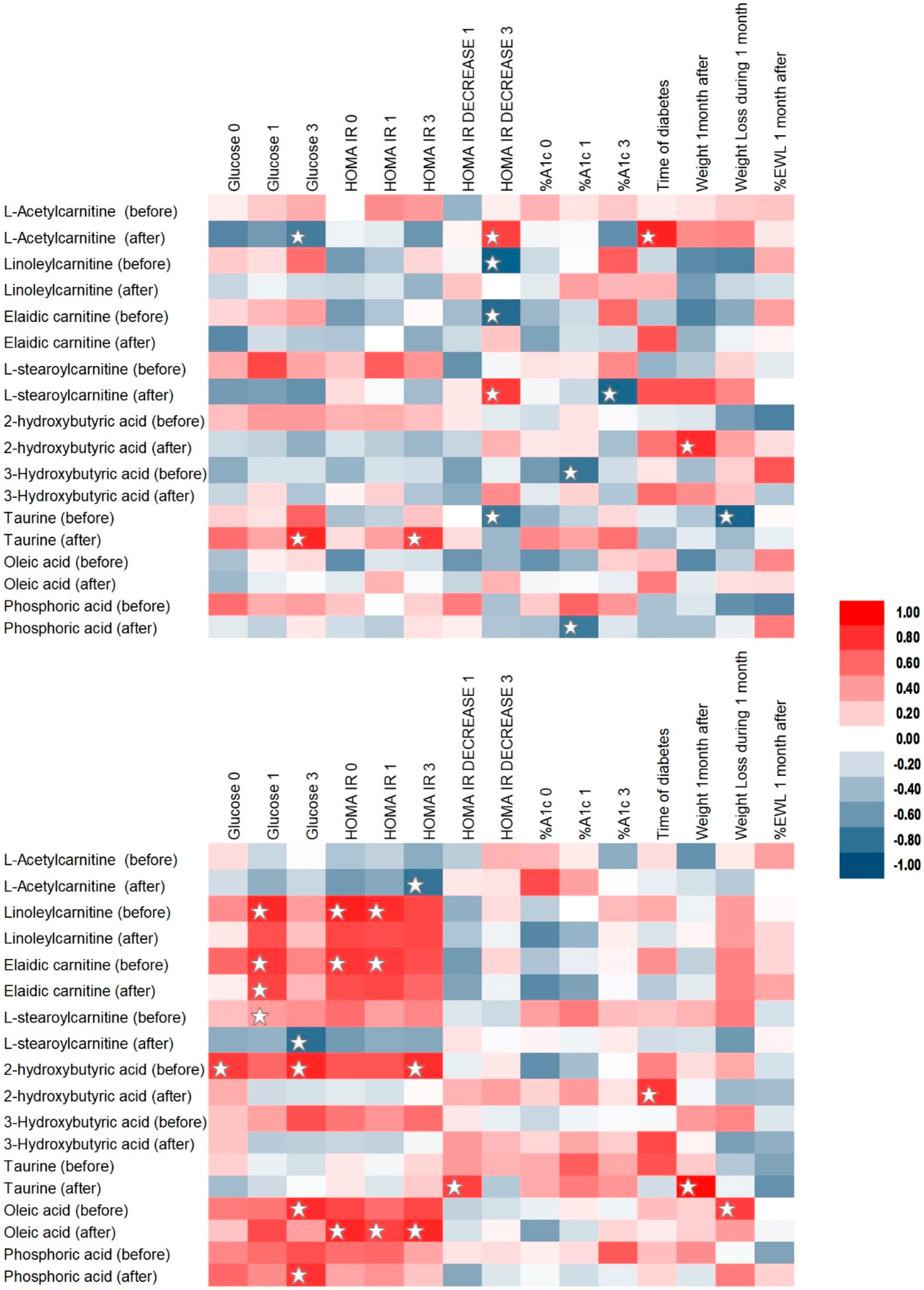
2.3. Correlation Analysis
2.4. Metabolic Pathways Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Study Participants
4.1.2. Samples Collection
4.1.3. Chemicals
4.2. Methods
4.2.1. Biochemical Measurements
4.2.2. Metabolic fingerprinting by LC-MS and GC-MS
4.2.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-HB | α-hydroxybutyric acid/2-hydroxybutyric acid |
| β-HB | β-hydroxybutyric acid/3-hydroxybutyric acid |
| acetyl-CoA | acetyl coenzyme A |
| ATP | Adenosine triphosphate |
| BCAAs | branched-chain amino acids |
| BMI | body mass index |
| CLR | C-type lectin receptor |
| (%)EWL | (%) excess weight loss |
| FA | fatty acid |
| FAA | fatty acid amides |
| FC | fold change |
| FDR | false discovery rate procedure |
| GC | gas chromatography |
| HbA1c | hemoglobin A1c (glycated hemoglobin) |
| HDL | high-density lipoprotein |
| HOMA | homeostatic model assessment |
| IR | insulin resistance |
| LC | liquid chromatography |
| LDL | low-density lipoprotein |
| (L)PE | (lyso)phosphatidylethanolamine |
| (L)PC | (lyso)phosphatidylcholine |
| LPS | lipopolysaccharide |
| LSG | laparoscopic sleeve gastrectomy |
| Mfn1 | Mitofusin 1 |
| MS | mass spectrometry |
| NAD | nicotinamide adenine dinucleotide |
| NADH | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| Nrf1 | nuclear respiratory factor 1 |
| OGTT | oral glucose tolerance test |
| PBMC | peripheral blood mononuclear cell |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| Q-TOF | quadrupole time-of-flight (mass spectrometer) |
| RONS | reactive oxygen and nitrogen species |
| ROS | reactive oxygen species |
| RYGB | Roux-en-Y gastric bypass |
| SCFA | short-chain fatty acids |
| SM | sphingomyelins |
| TCA | The citric acid cycle (CAC)/the tricarboxylic acid (TCA) cycle/Krebs cycle |
| TG | triglyceride |
| T2DM | type 2 diabetes mellitus |
References
- Kamvissi-Lorenz, V.; Raffaelli, M.; Bornstein, S.; Mingrone, G. Role of the Gut on Glucose Homeostasis: Lesson Learned from Metabolic Surgery. Curr. Atheroscler. Rep. 2017, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Almalki, O. Mechanism of diabetes control after metabolic surgery. Ann. Laparosc. Endosc. Surg. 2017, 2, 128. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Varela, J.E. Bariatric surgery for obesity and metabolic disorders: State of the art. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Celiker, H. A new proposed mechanism of action for gastric bypass surgery: Air hypothesis. Med. Hypotheses 2017, 107, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.M.; Toumi, Z.; Date, R.S. Surgical cure for type 2 diabetes by foregut or hindgut operations: A myth or reality? A systematic review. Surg. Endosc. 2017, 31, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.M.; Durda, M.A.; Stoicea, N.; Cavus, O.; Sahin, L.; Rogers, B. The Impact of Bariatric Surgery on Type 2 Diabetes Mellitus and the Management of Hypoglycemic Events. Front. Endocrinol. (Lausanne) 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Samczuk, P.; Luba, M.; Godzien, J.; Mastrangelo, A.; Hady, H.R.; Dadan, J.; Barbas, C.; Gorska, M.; Kretowski, A.; Ciborowski, M. “Gear mechanism” of bariatric interventions revealed by untargeted metabolomics. J. Pharm. Biomed. Anal. 2018, 151, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Samczuk, P.; Ciborowski, M.; Kretowski, A. Application of Metabolomics to Study Effects of Bariatric Surgery. J. Diabetes Res. 2018, 2018, 6270875. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Almulaifi, A.; Tsou, J.J.; Ser, K.H.; Lee, Y.C.; Chen, S.C. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: Predicting the success by ABCD score. Surg. Obes. Relat. Dis. 2015, 11, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Major, P.; Wysocki, M.; Pędziwiatr, M.; Małczak, P.; Pisarska, M.; Budzyński, A. Laparoscopic sleeve gastrectomy for the treatment of diabetes mellitus type 2 patients—Single center early experience. Gland Surg. 2016, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; le Roux, C.W.; Kokkinos, A. The role of bariatric surgery to treat diabetes: Current challenges and perspectives. BMC Endocr. Disord. 2017, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Benaiges, D.; Flores-Le Roux, J.A.; Pedro-Botet, J.; Chillaron, J.J.; Renard, M.; Parri, A.; Ramon, J.M.; Pera, M.; Goday, A. Sleeve gastrectomy and Roux-en-Y gastric bypass are equally effective in correcting insulin resistance. Int. J. Surg. 2013, 11, 309–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. α-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed]
- Cuperlovic-Culf, M. Machine Learning Methods for Analysis of Metabolic Data and Metabolic Pathway Modeling. Metabolites 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Franco-Obregon, A.; Gilbert, J.A. The Microbiome-Mitochondrion Connection: Common Ancestries, Common Mechanisms, Common Goals. mSystems 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Saint-Georges-Chaumet, Y.; Edeas, M. Microbiota-mitochondria inter-talk: Consequence for microbiota-host interaction. Pathog. Dis. 2016, 74, ftv096. [Google Scholar] [CrossRef] [PubMed]
- Dankel, S.N.; Staalesen, V.; Bjorndal, B.; Berge, R.K.; Mellgren, G.; Burri, L. Tissue-specific effects of bariatric surgery including mitochondrial function. J. Obes. 2011, 2011, 435245. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.; Mulya, A.; Fealy, C.E.; Huang, H.; Mosinski, J.D.; Pagadala, M.R.; Shimizu, H.; Batayyah, E.; Schauer, P.R.; Brethauer, S.A.; et al. Effect of Roux-en-Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Black, S.M. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 2009, 6, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Pekala, J.; Patkowska-Sokola, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochynski, S.; Librowski, T. L-carnitine—Metabolic functions and meaning in humans life. Curr. Drug Metab. 2011, 12, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Cattaneo, D.; Loriga, G.; Ledda, F.; Motterlini, N.; Gherardi, G.; Orisio, S.; Remuzzi, G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: Effects of acetyl-L-carnitine therapy. Hypertension 2009, 54, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.M.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, V.; Infantino, V. Citrate—New functions for an old metabolite. Biol. Chem. 2014, 395, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. beta-hydroxybutyrate: Much more than a metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.L.; Chevallier, J.M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2018. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Dore, J.; Clement, K. The importance of the gut microbiota after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.P.; Mota, M.; Martins, F.O.; Nogueira, C.; Goncalves, T.; Carneiro, T.; Pinto, J.; Duarte, D.; Barros, A.S.; Jones, J.G.; et al. Intestinal Microbial and Metabolic Profiling of Mice Fed with High-Glucose and High-Fructose Diets. J. Proteome Res. 2018, 17, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Moto, M.; Uchida, N.; He, F.; Hashizume, N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J. Clin. Biochem. Nutr. 2012, 51, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamboa, J.L.; Billings, F.T.; Bojanowski, M.T.; Gilliam, L.A.; Yu, C.; Roshanravan, B.; Roberts, L.J.; Himmelfarb, J.; Ikizler, T.A.; Brown, N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Sharma, A.; Wu, W.; Gomi, R.; Sung, B.; Hospodsky, D.; Angenent, L.T.; Worgall, S. The fermentation product 2,3-butanediol alters P. aeruginosa clearance, cytokine response and the lung microbiome. ISME J. 2016, 10, 2978–2983. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; da Silva, E.J.N.; Duque, T.M.; Zaia, A.A.; Ferraz, C.C.R.; de Almeida, J.F.A.; de Almeida Gomes, B.P.F. Antimicrobial and cytotoxic effects of phosphoric acid solution compared to other root canal irrigants. J. Appl. Oral Sci. 2015, 23, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Grander, C. Microbiota and diabetes: An increasingly relevant association. Pol. Arch. Intern. Med. 2018, 128, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Godzien, J.; Ciborowski, M.; Martinez-Alcazar, M.P.; Samczuk, P.; Kretowski, A.; Barbas, C. Rapid and Reliable Identification of Phospholipids for Untargeted Metabolomics with LC-ESI-QTOF-MS/MS. J. Proteome Res. 2015, 14, 3204–3216. [Google Scholar] [CrossRef] [PubMed]


| Time Frame | Biochemical and Clinical Parameters | Quicker Remission | Slower Remission | p-Value |
|---|---|---|---|---|
| Age (years) | 47 ± 10.3 | 51 ± 11.4 | ns | |
| Sex–F/M | 4/7 | 4/5 | - | |
| Body Mass Index (BMI) before | 49 ± 4.5 | 51 ± 8.3 | ns | |
| BMI 1 month after | 42 ± 3.5 | 46 ± 8.1 | ns | |
| BMI 3 months after | 37 ± 3.6 | 43 ± 7.6 | ns | |
| Excess weight loss (EWL, %) 1 month after | 21 ± 5.9 | 18 ± 4.8 | ns | |
| Excess weight loss (EWL, %) 3 months after | 29 ± 9.8 | 29 ± 7.4 | ns | |
| Weight loss after 1 month (kg) | 16 ± 4.7 | 15 ± 2.8 | ns | |
| Time of type 2 diabetes (T2DM) lasting (months) | 30 ± 59.3 | 24 ± 16.5 | ns | |
| Before surgery | Glucose (mg/dL) | 158 ± 62.6 | 153 ± 36.1 | ns |
| Insulin (pmol/L) | 46 ± 39.4 | 36 ± 13.8 | ns | |
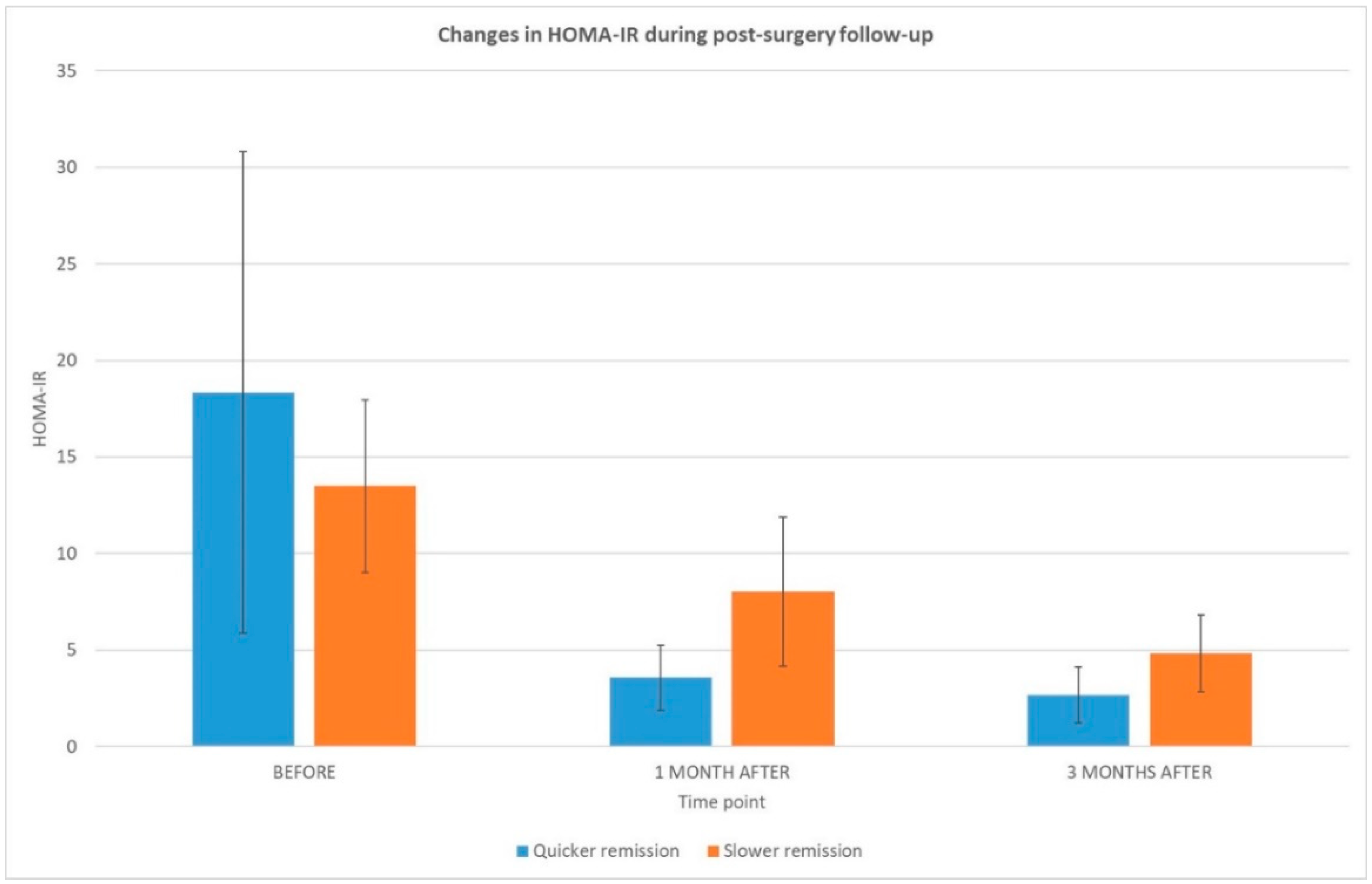
| HOMA-IR | 18.3 ± 12.5 | 13.5 ± 4.5 | ns | |
| Cholesterol (mg/dL) | 198 ± 43.4 | 186 ± 27.7 | ns | |
| LDL (mg/dL) | 147 ± 43.4 | 114 ± 29.2 | ns | |
| HDL (mg/dL) | 50 ± 22.8 | 42 ± 6.6 | ns | |
| TG (mg/dL) | 150 ± 55.2 | 154 ± 51.5 | ns | |
| A1c (%) | 6.88 ± 1.3 | 6.26 ± 0.7 | ns | |
| 1 month post-surgery | Glucose (mg/dL) | 102 ± 20.3 | 118 ± 22.0 | ns |
| Insulin (pmol/L) | 13 ± 7.7 | 26 ± 11.1 | 0.011 | |
| HOMA-IR | 3.6 ± 1.7 | 8.0 ± 3.9 | 0.029 | |
| HOMA-IR decrease (%) | 76 ± 8.9 | 42 ± 16.2 | 0.000 | |
| Cholesterol (mg/dL) | 190 ± 43.5 | 197 ± 28.7 | ns | |
| LDL (mg/dL) | 126 ± 38.0 | 135 ± 30.0 | ns | |
| HDL (mg/dL) | 48 ± 33.5 | 59 ± 43.9 | ns | |
| TG (mg/dL) | 130 ± 45.4 | 149 ± 75.5 | ns | |
| A1c (%) | 6.3 ± 1.0 | 5.9 ± 0.4 | ns | |
| 3 months post-surgery | Glucose (mg/dL) | 99 ± 17.7 | 108 ± 11.3 | ns |
| Insulin (pmol/L) | 10 ± 6.1 | 18 ± 8.5 | ns | |
| HOMA-IR | 2.67 ± 1.5 | 4.82 ± 2.0 | ns | |
| HOMA-IR decrease (%) | 80 ± 12.3 | 63 ± 13.9 | 0.011 | |
| Cholesterol (mg/dL) | 184 ± 46.8 | 188 ± 25.3 | ns | |
| LDL (mg/dL) | 128 ± 37.8 | 132 ± 35.4 | ns | |
| HDL (mg/dL) | 51 ± 19.5 | 47 ± 11.8 | ns | |
| TG (mg/dL) | 125 ± 37.8 | 139 ± 28.7 | ns | |
| A1c (%) | 5.8 ± 0.6 | 6.5 ±1.3 | ns |
| Metabolite | Quicker Remission | Slower Remission |
|---|---|---|
| Lyso PC 14:0 (+) | 0.54 ** | 0.56 * |
| Lyso PC 20:5sn-2 (+) | 0.50 **** | 0.61 * |
| Lyso PC 20:3 (+) | 0.41 **** | 0.61 * |
| Lyso PC 20:5sn-1 (+) | 0.50 **** | 0.61 * |
| PC 30:0 (+) | 0.49 ** | 0.60 * |
| PC 32:1 (+) | 0.56 ** | 0.58 ** |
| PC 34:4 (+) | 0.48 ** | 0.54 * |
| PC 40:5 (+) | 0.42 ** | 0.51 * |
| Lyso PE 18:1 (−) | 0.72 * | 0.72 * |
| Lyso PE 20:5 (+/−) | 0.54 ** | 0.55 * |
| PE 16:0/20:5 (−) | 0.52 ** | 0.56 * |
| arachidonic acid (−) | 1.34 ** | 1.31 ** |
| Metabolite | 0 vs. 1 Month | Quicker vs. Slower Remission | ||
|---|---|---|---|---|
| Quicker Remission | Slower Remission | Before Surgery | 1 Month Post-Surgery | |
| l-Acetylcarnitine(+) | 2.24 * | ns | ns | 2.32 * |
| Linoleylcarnitine (+) | 1.45 ** | ns | ns | ns |
| Elaidic carnitine (+) | 1.55 ** | 1.46 * | ns | ns |
| l-stearoylcarnitine (+) | ns | 0.60 * | ns | ns |
| Lactic acid (−) | 0.58 * | ns | ns | ns |
| Uric acid (−) | 1.26 * | ns | ns | ns |
| Oleic acid (−) | 1.40 ** | 1.39 * | ns | 0.67 * |
| Oleamide (+) | ns | 0.62 **** | ns | ns |
| Stearamide (+) | ns | 0.75 * | ns | ns |
| Lyso PE 16:0 (−) | ns | ns | 0.56 * | ns |
| Lyso PE P-16:0 (+) | ns | ns | 0.75 * | ns |
| Lyso PE 18:0 (+/−) | 0.72 * | ns | 066 * | 0.65 * |
| Lyso PE 18:1 (+/−) | ns | ns | 0.66 * | 0.63 * |
| Lyso PE 18:2 (+/−) | 0.61 ** | ns | ns | 0.63 * |
| Lyso PE 20:0 (−) | ns | ns | ns | 0.61 * |
| Lyso PE 20:3 (+/−) | 0.51 * | ns | 0.63 * | 0.63 * |
| Lyso PE 20:4 (−) | ns | ns | ns | 0.71 * |
| Lyso PE 22:5 (−) | ns | ns | 0.55 * | 0.56 * |
| PE 18:1/16:0 (−) | ns | 0.74 * | ns | ns |
| PE 18:2/16:0 (−) | 0.61 * | ns | ns | ns |
| PE 16:0/20:5 (−) | ns | ns | 0.70 * | ns |
| PE 18:0/18:2 (−) | 0.50 * | ns | ns | ns |
| PE 18:0/20:3 (−) | 0.54 **** | ns | ns | ns |
| Lyso PC 14:0 (−) | 0.65 * | ns | ns | 0.57 * |
| Lyso PC 15:0 (−) | ns | ns | 0.75 * | 0.69 * |
| Lyso PC 16:0 (−) | ns | ns | 0.64 * | ns |
| Lyso PC 16:1 (+) | 0.66 * | ns | ns | ns |
| Lyso PC 17:1 (+) | ns | ns | 0.71 * | ns |
| Lyso PC 18:0 (+/−) | 0.71 ** | ns | 0.72 * | 0.59 * |
| Lyso PC 18:2 (+/−) | 0.73 * | ns | ns | 0.69 * |
| Lyso PC 20:1 (−) | ns | ns | 0.76 * | ns |
| Lyso PC 20:2 (+/−) | 0.70 ** | ns | 0.70 * | ns |
| Lyso PC 20:3 (+/−) | 0.60 * | ns | ns | ns |
| Lyso PC 22:4 (+) | 0.70 * | ns | ns | ns |
| Lyso PC 22:5 (+) | 0.61 ** | ns | 1.50 * | ns |
| PC 30:0 (+) | ns | ns | ns | 0.64 * |
| PC 32:2 (+) | 0.49 ** | ns | ns | ns |
| PC 34:3 (+) | 0.56 ** | ns | ns | ns |
| PC 18:2/17:0 (−) | 0.68 ** | ns | ns | ns |
| PC 38:5 (+) | 0.27 * | ns | ns | ns |
| PC 22:5/16:0 (−) | ns | 1.27 * | ns | ns |
| SM 33:2(−) | ns | 1.32 *** | ns | ns |
| C14:1 sphingolipid (+) | 0.68 **** | ns | 0.49 * | ns |
| Sphingosine (+) | ns | ns | ns | 1.40 * |
| Choline (+) | ns | 0.70 * | 0.49 * | ns |
| Lueucine/isoleucine (+) | ns | 0.62 **** | ns | ns |
| Piperine (+) | ns | 0.58 * | 0.48 * | ns |
| Oxotetradecenoic acid or hydroxytetradecenoic acid (−) | 2.06 * | ns | ns | ns |
| Hydroperoxyoctadecadienoic acid (−) | 2.06 * | ns | ns | ns |
| Succinyldiaminopimelic acid (−) | ns | 0.75 **** | ns | ns |
| Hydroxyandrostanone sulfate (−) | 1.28 **** | 1.35 * | ns | ns |
| Androsterone sulfate (−) | 1.31 **** | ns | ns | ns |
| Hydroperoxylinoleic acid (−) | 0.55 * | ns | ns | ns |
| Taurine (−) | ns | ns | 0.43 * | ns |
| Metabolite | 0 vs. 1 Month | Quicker vs. Slower Remission | ||
|---|---|---|---|---|
| Quicker Remission | Slower Remission | Before Surgery | 1 Month Post-Surgery | |
| 2-hydroxybutyric acid | 1.69 * | ns | ns | 1.70 * |
| 3-hydroxybutyric acid | 7.32 ** | ns | ns | 4.76 ** |
| 2-ketoisocaproic acid | 1.97 ** | 1.68 * | ns | ns |
| phosphoric acid | 4.55 * | ns | ns | ns |
| fumaric acid | 1.91 * | ns | ns | ns |
| l-(+) lactic acid | ns | ns | 0.47 * | ns |
| citric acid | 2.75 * | ns | ns | 2.48 ** |
| linoleic acid | ns | ns | ns | 1.61 * |
| l-valine/norvaline | ns | 0.76 ** | ns | ns |
| l-leucine | ns | 0.70 ** | ns | ns |
| Isoleucine/norleucine | ns | 0.76 * | ns | ns |
| l-threonine | ns | 0.73 * | ns | ns |
| l-methionine | ns | 0.68 ** | ns | ns |
| Phenylalanine | ns | 0.60 * | ns | ns |
| l-tyrosine | ns | 0.62 ** | ns | ns |
| l-tryptophan | ns | 0.63 ** | ns | ns |
| l-alanine | ns | ns | 0.63 * | ns |
| N-methylalanine | 1.81 * | ns | ns | ns |
| Glycerol | 2.11 ** | 1.73 * | ns | ns |
| ribose | ns | 0.44 * | ns | ns |
| d-mannose/d-allose | ns | 0.69 * | ns | ns |
| Furanose (tagatose) | 0.47 ** | ns | ns | ns |
| 2,3-Butanediol, 2TMS derivative | ns | ns | 0.41 ** | ns |
| Group | Pathway | p-Value | FDR | Impact |
|---|---|---|---|---|
| Quicker remission | Butanoate metabolism | 3.43 × 10−3 | 2.74 × 10−1 | 0.02 |
| Citrate cycle | 1.03 × 10−2 | 2.75 × 10−1 | 0.08 | |
| Taurine and hypotaurine metabolism | 1.03 × 10−2 | 2.75 × 10−1 | 0.36 | |
| Alanine, aspartate and glutamate metabolism | 1.47 × 10−2 | 2.94 × 10−1 | 0.06 | |
| Propanoate metabolism | 3.01 × 10−2 | 4.81 × 10−1 | 0.00 | |
| Galactose metabolism | 4.03 × 10−2 | 5.31 × 10−1 | 0.03 | |
| Synthesis and degradation of ketone bodies | 4.65 × 10−2 | 5.31 × 10−1 | 0.00 | |
| Slower remission | Aminoacyl-tRNA biosynthesis | 1.44 × 10−7 | 1.15 × 10−5 | 0.00 |
| Valine, leucine and isoleucine biosynthesis | 1.42 × 10−5 | 5.67 × 10−4 | 0.07 | |
| Nitrogen metabolism | 1.19 × 10−3 | 2.63 × 10−2 | 0.00 | |
| Valine, leucine and isoleucine degradation | 1.31 × 10−3 | 2.63 × 10−2 | 0.06 | |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 4.18 × 10−3 | 6.69 × 10−2 | 0.01 | |
| Propanoate metabolism | 8.74 × 10−3 | 1.17 × 10−1 | 0.00 | |
| Glycine, serine and threonine metabolism | 2.07 × 10−2 | 2.37 × 10−1 | 0.10 | |
| Taurine and hypotaurine metabolism | 2.48 × 10−2 | 2.48 × 10−1 | 0.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samczuk, P.; Hady, H.R.; Adamska-Patruno, E.; Citko, A.; Dadan, J.; Barbas, C.; Kretowski, A.; Ciborowski, M. In-and-Out Molecular Changes Linked to the Type 2 Diabetes Remission after Bariatric Surgery: An Influence of Gut Microbes on Mitochondria Metabolism. Int. J. Mol. Sci. 2018, 19, 3744. https://doi.org/10.3390/ijms19123744
Samczuk P, Hady HR, Adamska-Patruno E, Citko A, Dadan J, Barbas C, Kretowski A, Ciborowski M. In-and-Out Molecular Changes Linked to the Type 2 Diabetes Remission after Bariatric Surgery: An Influence of Gut Microbes on Mitochondria Metabolism. International Journal of Molecular Sciences. 2018; 19(12):3744. https://doi.org/10.3390/ijms19123744
Chicago/Turabian StyleSamczuk, Paulina, Hady Razak Hady, Edyta Adamska-Patruno, Anna Citko, Jacek Dadan, Coral Barbas, Adam Kretowski, and Michal Ciborowski. 2018. "In-and-Out Molecular Changes Linked to the Type 2 Diabetes Remission after Bariatric Surgery: An Influence of Gut Microbes on Mitochondria Metabolism" International Journal of Molecular Sciences 19, no. 12: 3744. https://doi.org/10.3390/ijms19123744






