Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells
Abstract
:1. Introduction
2. Results
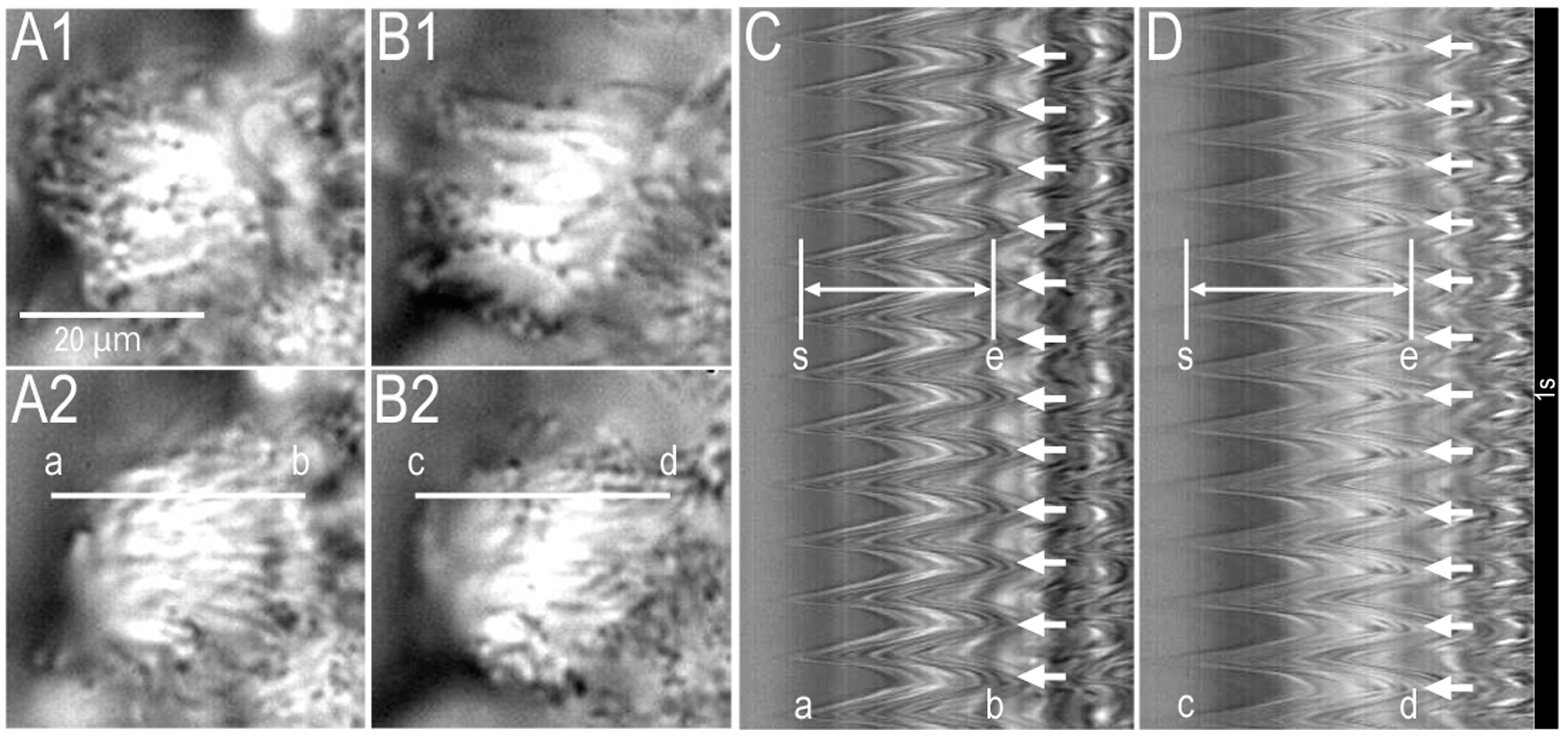
2.1. Video Images of cHNECs Activated by Daidzein
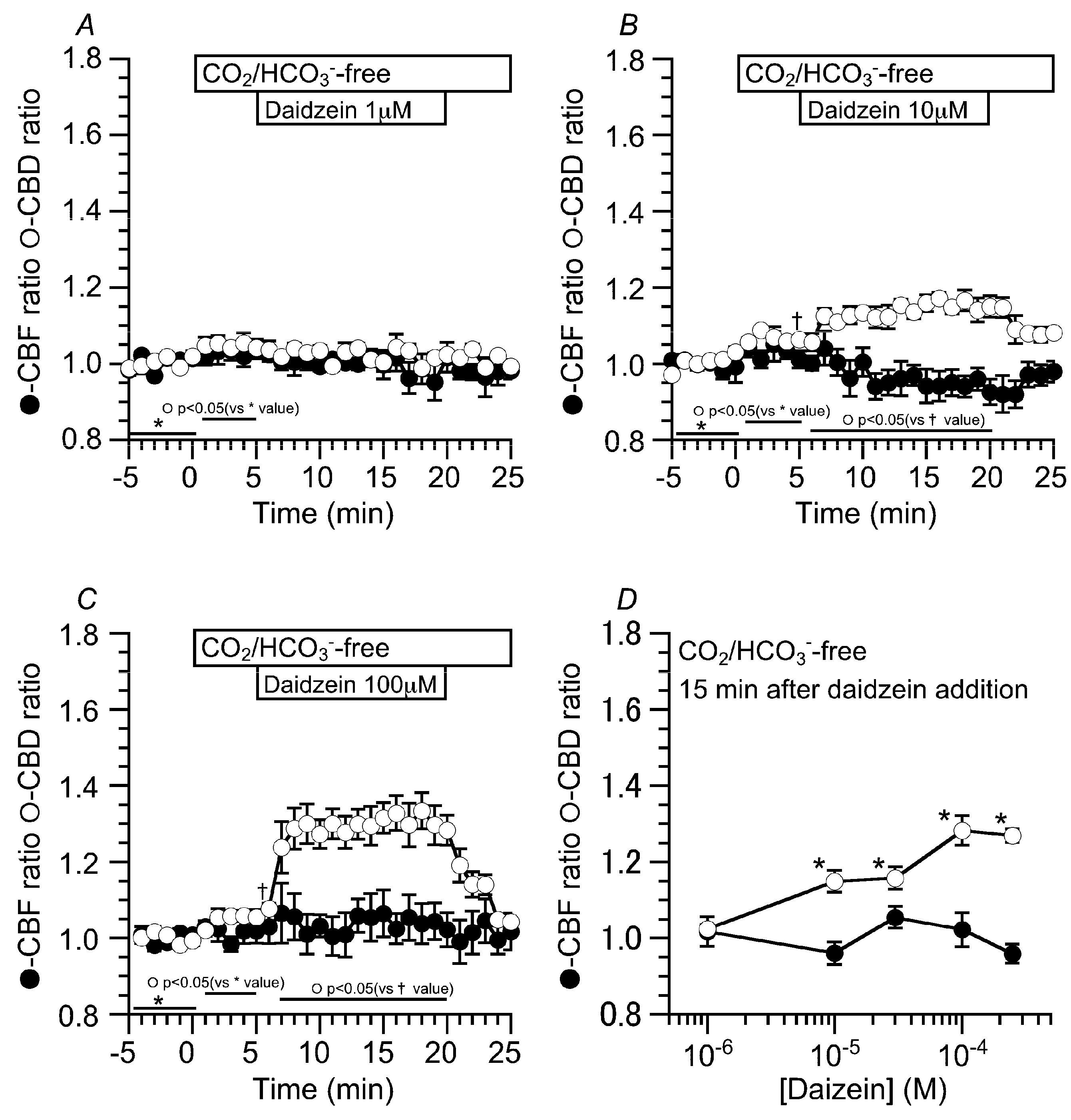
2.2. Effects of Daidzein on CBD and CBF
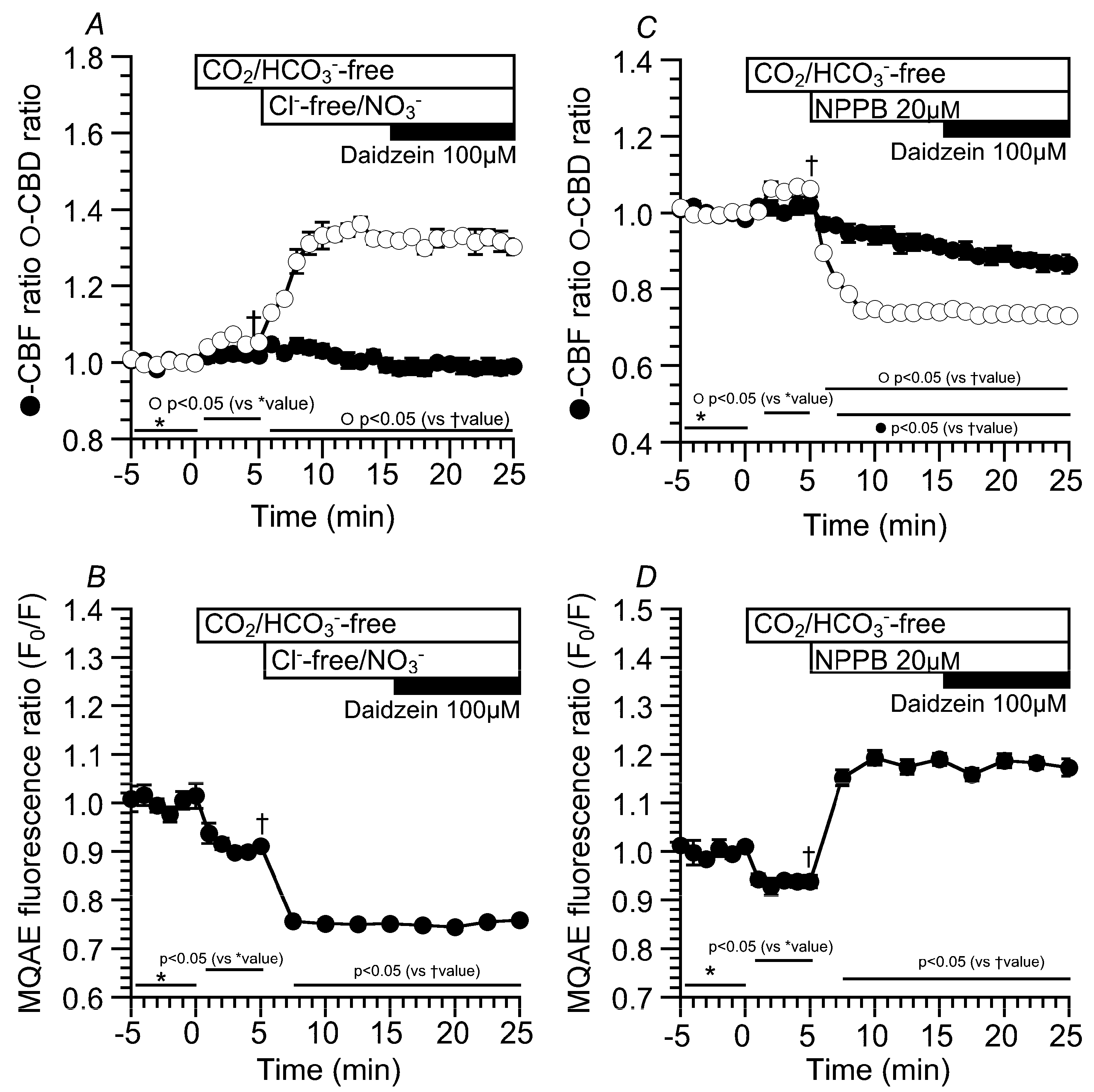
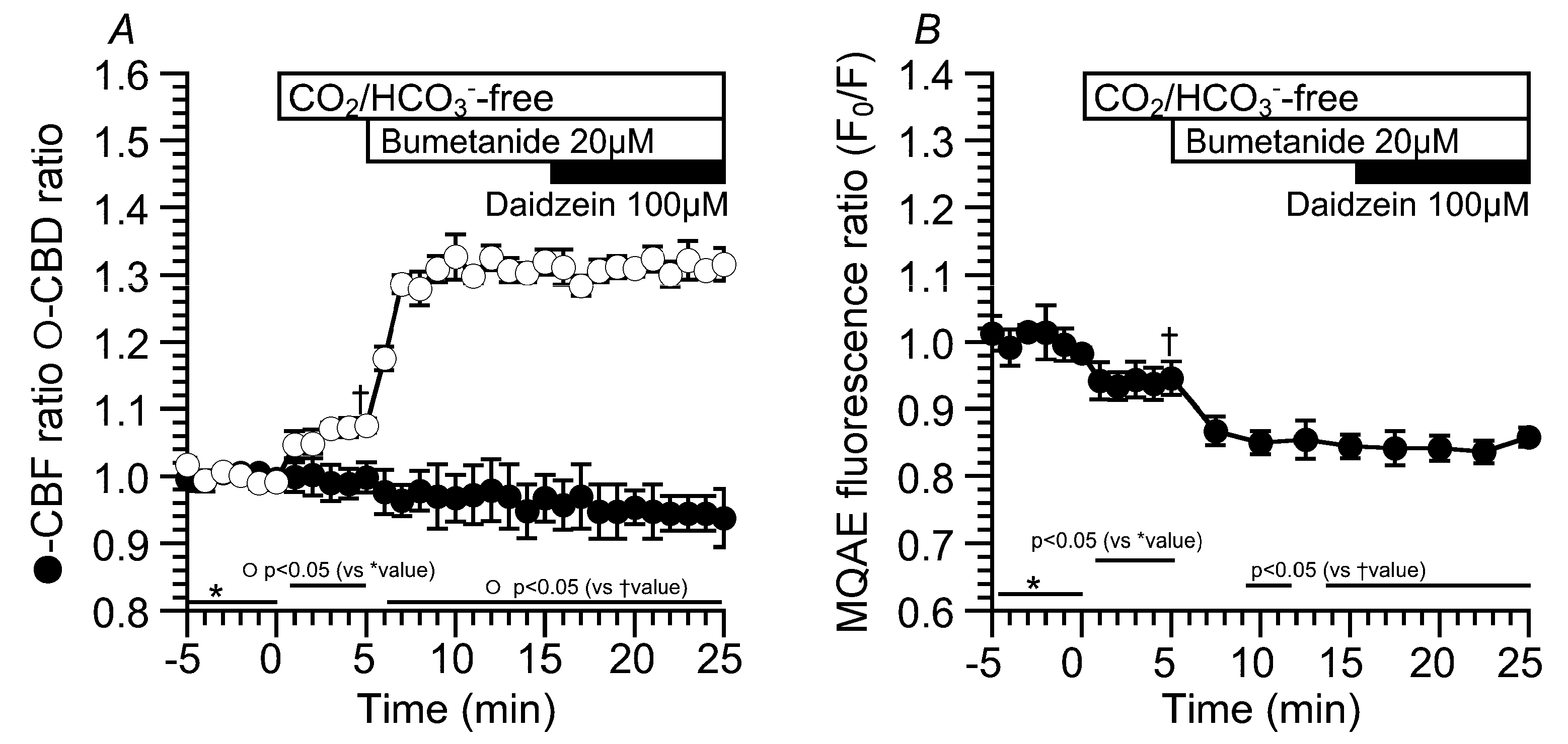
2.3. Effects of Daidzein on [Cl−]i, CBD, and CBF
2.4. Latex Microbeads Movement Driven by the Beating Cilia of cHNECs
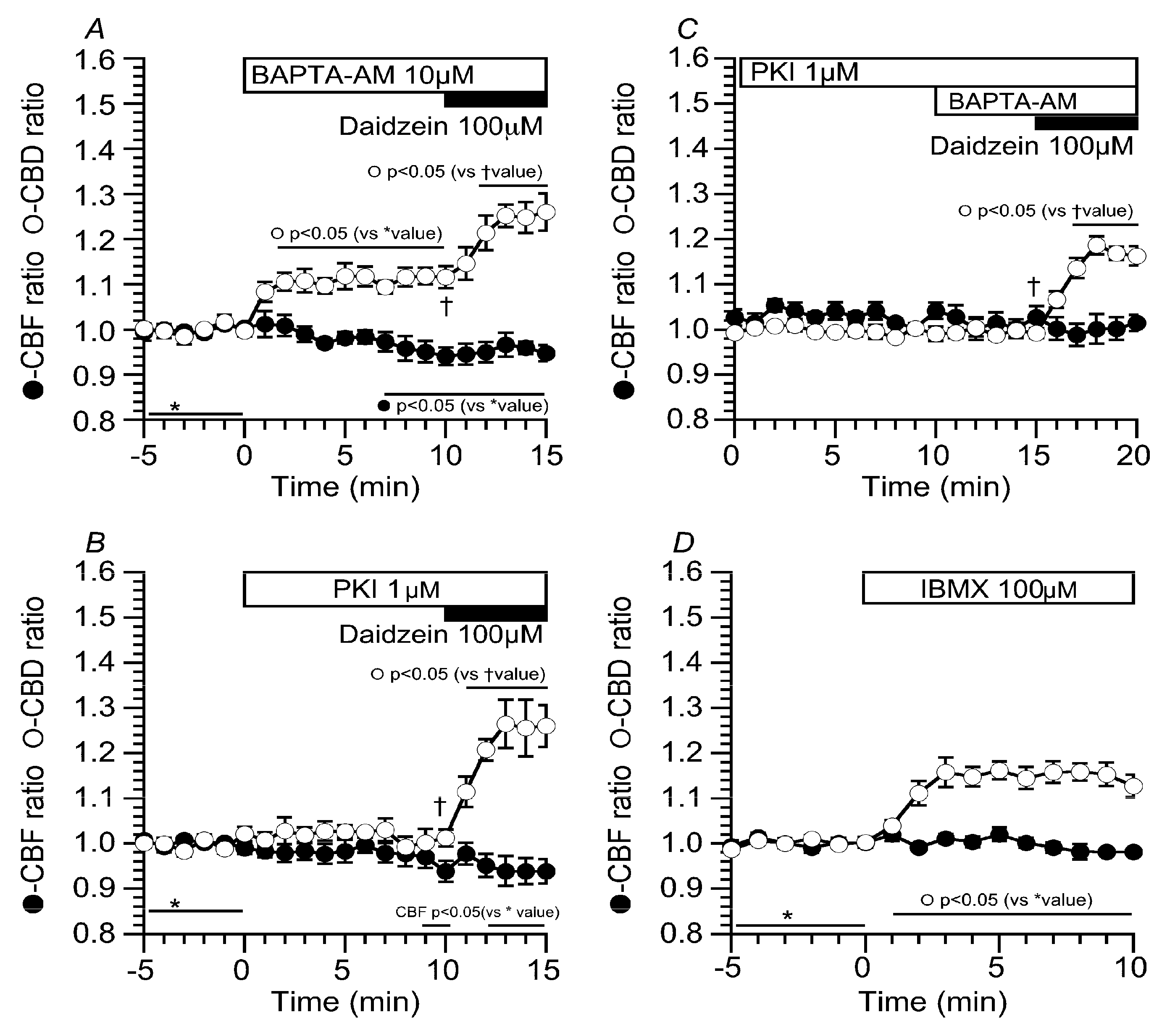
2.5. Effects of Ca2+ and cAMP on CBD and CBF Stimulated by Daidzein
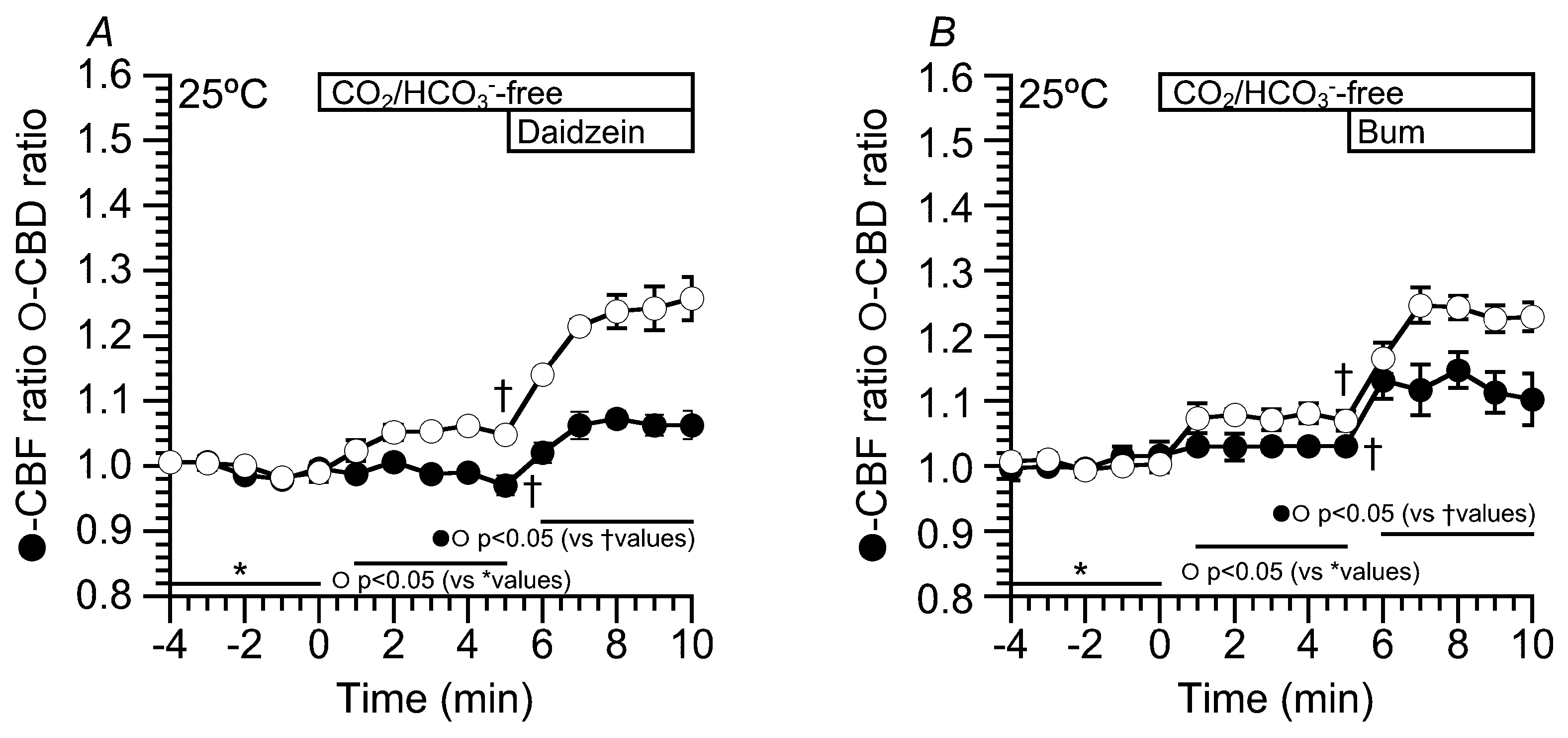
2.6. Effects of Daidzein on CBD and CBF at 25 °C
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Solution and Chemicals
4.3. Cell Preparation and Culture
4.4. CBD and CBF Measurements
4.5. Measurement of [Cl−]i
4.6. Observation of Latex Microbead Transport in cHNECs
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afzelius, B.A. Cilia-related diseases. J. Pathol. 2004, 204, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, A.; Salathe, M.; O’Riordan, T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996, 154, 1868–1902. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Daniels, L.A.; Davis, S.D.; Zariwala, M.A.; Leigh, M.W. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am. J. Respir. Crit. Care Med. 2013, 188, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Komatani-Tamiya, N.; Daikoku, E.; Takemura, Y.; Shimamoto, C.; Nakano, T.; Iwasaki, Y.; Kohda, Y.; Matsumura, H.; Marunaka, Y.; Nakahari, T. Procaterol-stimulated increases in ciliary bend amplitude and ciliary beat frequency in mouse bronchioles. Cell. Physiol. Biochem. 2012, 29, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Nakano, T.; Sano, K.I.; Inui, T.; Marunaka, Y.; et al. A low [Ca2+]i-induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflugers Arch. 2017, 469, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Inui, T.; Marunaka, Y.; Nakahari, T. [Ca(2+)]i modulation of cAMP-stimulated ciliary beat frequency via PDE1 in airway ciliary cells of mice. Exp. Physiol. 2018, 103, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Ikeuchi, Y.; Sumiya, M.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Marunaka, Y.; Nakahari, T. Seihai-to (TJ-90)-Induced Activation of Airway Ciliary Beatings of Mice: Ca(2+) Modulation of cAMP-Stimulated Ciliary Beatings via PDE1. Int. J. Mol. Sci. 2018, 19, 658. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamto, C.; Matsumura, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Carbocisteine stimulated an increase in ciliary bend angle via a decrease in [Cl-]i in mouse airway cilia. Pflugers Arch. 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K. [Ca2+]i modulation of Cl- content controls cell volume in single salivary acinar cells during fluid secretion. Am. J. Physiol. 1990, 259, C998–C1004. [Google Scholar] [CrossRef] [PubMed]
- Treharne, K.J.; Marshall, L.J.; Mehta, A. A novel chloride-dependent GTP-utilizing protein kinase in plasma membranes from human respiratory epithelium. Am. J. Physiol. 1994, 267, L592–L601. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, C.; Umegaki, E.; Katsu, K.; Kato, M.; Fujiwara, S.; Kubota, T.; Nakahari, T. [Cl−]i modulation of Ca2+-regulated exocytosis in ACh-stimulated antral mucous cells of guinea pig. Am. J. Physiol. 2007, 293, G824–G837. [Google Scholar] [CrossRef] [PubMed]
- Conger, B.T.; Zhang, S.; Skinner, D.; Hicks, S.B.; Sorscher, E.J.; Rowe, S.M.; Woodworth, B.A. Comparison of cystic fibrosis transmembrane conductance regulator (CFTR) and ciliary beat frequency activation by the CFTR Modulators Genistein, VRT-532, and UCCF-152 in primary sinonasal epithelial cultures. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Niisato, N.; Ito, Y.; Marunaka, Y. Activation of Cl− channel and Na+/K+/2Cl− cotransporter in renal epithelial A6 cells by flavonoids: Genistein, daidzein, and apigenin. Biochem. Biophys. Res. Commun. 1999, 254, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y. Actions of quercetin, a flavonoid, on ion transporters: Its physiological roles. Ann. N. Y. Acad. Sci. 2017, 1398, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, A.; Dolovich, M.B. Airway Epithelial Cell Cilia and Obstructive Lung Disease. Cells 2016, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Inui, T.; Nakahari, T.; Marunaka, Y. Measurement of [Cl(−)]i unaffected by the cell volume change using MQAE-based two-photon microscopy in airway ciliary cells of mice. J. Physiol. Sci. 2018, 68, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Munkonge, F.; Alton, E.W.; Andersson, C.; Davidson, H.; Dragomir, A.; Edelman, A.; Farley, R.; Hjelte, L.; McLachlan, G.; Stern, M.; et al. Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators. J. Cyst. Fibros 2004, 3, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marunaka, Y. Hormonal and osmotic regulation of NaCl transport in renal distal nephron epithelium. Jpn. J. Physiol. 1997, 47, 499–511. [Google Scholar] [CrossRef]
- Miyazaki, H.; Shiozaki, A.; Niisato, N.; Marunaka, Y. Physiological significance of hypotonicity-induced regulatory volume decrease: Reduction in intracellular Cl- concentration acting as an intracellular signaling. Am. J. Physiol. Renal Physiol. 2007, 292, F1411–F1417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Smith, N.; Schuster, D.; Azbell, C.; Sorscher, E.J.; Rowe, S.M.; Woodworth, B.A. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am. J. Rhinol. Allergy 2011, 25, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Skinner, D.; Hicks, S.B.; Bevensee, M.O.; Sorscher, E.J.; Lazrak, A.; Matalon, S.; McNicholas, C.M.; Woodworth, B.A. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS ONE 2014, 9, e104090. [Google Scholar] [CrossRef] [PubMed]
- Azbell, C.; Zhang, S.; Skinner, D.; Fortenberry, J.; Sorscher, E.J.; Woodworth, B.A. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol. Head Neck Surg. 2010, 143, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiima-Kinoshita, C.; Min, K.Y.; Hanafusa, T.; Mori, H.; Nakahari, T. Beta 2-adrenergic regulation of ciliary beat frequency in rat bronchiolar epithelium: Potentiation by isosmotic cell shrinkage. J. Physiol. 2004, 554, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, K.; Niisato, N.; Taruno, A.; Marunaka, Y. Simulation of Cl(−) Secretion in Epithelial Tissues: New Methodology Estimating Activity of Electro-Neutral Cl(−) Transporter. Front Physiol. 2015, 6, 370. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J.; Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskel. 1987, 8, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J. Control of flagellar bending: A new agenda based on dynein diversity. Cell Motil. Cytoskel. 1994, 28, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiozaki, A.; Miyazaki, H.; Niisato, N.; Nakahari, T.; Iwasaki, Y.; Itoi, H.; Ueda, Y.; Yamagishi, H.; Marunaka, Y. Furosemide, a blocker of Na+/K+/2Cl− cotransporter, diminishes proliferation of poorly differentiated human gastric cancer cells by affecting G0/G1 state. J. Physiol. Sci. 2006, 56, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Shpetner, H.S.; Paschal, B.M.; Vallee, R.B. Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP 1C). J. Cell Biol. 1988, 107, 1001–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foskett, J.K.; Wong, D. Calcium oscillations in parotid acinar cells induced by microsomal Ca(2+)-ATPase inhibition. J. Cell Biol. 1992, 262, C656–C663. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci. Biotechnol. Biochem. 2013, 77, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Kuremoto, T.; Kogiso, H.; Yasuda, M.; Inui, T.; Murakami, K.; Hirano, S.; Ikeuchi, Y.; Hosigi, S.; Inui, T.; Marunaka, Y.; et al. Spontaneous oscillation of the ciliary beat frequency regulated by release of Ca2+ from intracellular stores in mouse nasal epithelia. Biochem. Biophys. Res. Commun. 2018, 507, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Brighton, L.E.; Carson, J.L.; Fischer, W.A., 2nd; Jaspers, I. Culturing of human nasal epithelial cells at the air liquid interface. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Niisato, N.; Miyazaki, H.; Hama, T.; Dejima, K.; Hisa, Y.; Marunaka, Y. Epithelial ion transport of human nasal polyp and paranasal sinus mucosa. Am. J. Respir. Cell Mol. Biol. 2007, 36, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Niisato, N.; Miyazaki, H.; Iwasaki, Y.; Hama, T.; Dejima, K.; Hisa, Y.; Marunaka, Y. Epithelial Na+ channel and ion transport in human nasal polyp and paranasal sinus mucosa. Biochem. Biophys. Res. Commun. 2007, 362, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, P.; Sanderson, M.J. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, I.M.; Liedtke, W.; Sanderson, M.J.; Valverde, M.A. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 12611–12616. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inui, T.-a.; Yasuda, M.; Hirano, S.; Ikeuchi, Y.; Kogiso, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 3754. https://doi.org/10.3390/ijms19123754
Inui T-a, Yasuda M, Hirano S, Ikeuchi Y, Kogiso H, Inui T, Marunaka Y, Nakahari T. Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells. International Journal of Molecular Sciences. 2018; 19(12):3754. https://doi.org/10.3390/ijms19123754
Chicago/Turabian StyleInui, Taka-aki, Makoto Yasuda, Shigeru Hirano, Yukiko Ikeuchi, Haruka Kogiso, Toshio Inui, Yoshinori Marunaka, and Takashi Nakahari. 2018. "Daidzein-Stimulated Increase in the Ciliary Beating Amplitude via an [Cl−]i Decrease in Ciliated Human Nasal Epithelial Cells" International Journal of Molecular Sciences 19, no. 12: 3754. https://doi.org/10.3390/ijms19123754



