Influence of Implant Material and Surface on Differentiation and Proliferation of Human Adipose-Derived Stromal Cells
Abstract
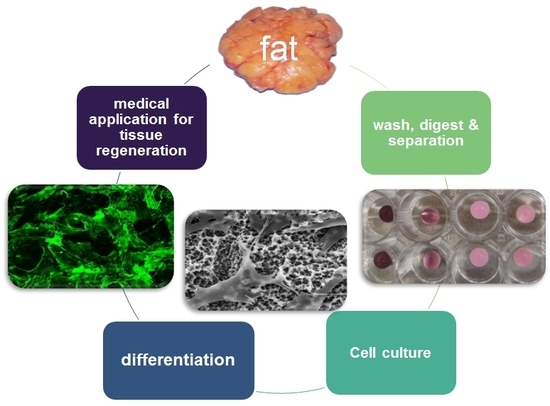
:1. Introduction
2. Results
2.1. Cell Viability
2.2. Expression of Stem Cell and Osteogenic Marker
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethical Approval
4.2. Isolation of Primary Human Cell Cultures
4.3. Main Cell Culture
4.4. Cell Viability
4.5. Protein Expression Analysis
4.6. RNA Extraction and Real-Time qPCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| hADSC | Human adipose-derived stromal cells |
| hOB | Human osteoblasts |
| HGF | Human gingival fibroblasts |
References
- Mercado, F.; Hamlet, S.; Ivanovski, S. Regenerative surgical therapy for peri-implantitis using deproteinized bovine bone mineral with 10% collagen, enamel matrix derivative and Doxycycline-A prospective 3-year cohort study. Clin. Oral Implants Res. 2018, 29, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Roos-Jansaker, A.M.; Persson, G.R. Surgical treatment of peri-implantitis lesions with or without the use of a bone substitute—A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Pandis, N.; Mustafa, K.; Nyengaard, J.R.; Stavropoulos, A. Bone tissue engineering in oral peri-implant defects in preclinical in vivo research: A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2018, 12, e336–e349. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Salvador, E.; Ruaro, M.E.; Melato, M.; Tromba, G.; Angerame, D.; & Bevilacqua, L. Bone regeneration with adipose derived stem cells in a rabbit model. J. Biomed. Res. 2018. [Google Scholar] [CrossRef]
- Valenti, M.T.; Dalle Carbonare, L.; Mottes, M. Osteogenic Differentiation in Healthy and Pathological Conditions. Int. J. Mol. Sci. 2016, 18, 41. [Google Scholar] [CrossRef]
- Jung, S.; Kleineidam, B.; Kleinheinz, J. Regenerative potential of human adipose-derived stromal cells of various origins. J. Craniomaxillofac. Surg. 2015, 43, 2144–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Yang, Z.; Sun, S.; Huang, H.; Sun, X.; Wang, Z.; Zhang, Y.; Zhang, B. Adipose-derived stem cells modified genetically in vivo promote reconstruction of bone defects. Cytotherapy 2010, 12, 831–840. [Google Scholar] [CrossRef]
- Shiraishi, T.; Sumita, Y.; Wakamastu, Y.; Nagai, K.; Asahina, I. Formation of engineered bone with adipose stromal cells from buccal fat pad. J. Dent. Res. 2012, 91, 592–597. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Charnley, M.; Goren, T.; Guimond-Lischer, S.; Rottmar, M.; Maniura-Weber, K.; Spencer, D.D.; Reis, R.; Textor, M. Osteogenic differentiation of human mesenchymal stem cells in the absence of osteogenic supplements: A surface-roughness gradient study. Acta Biomater. 2015, 28, 64–75. [Google Scholar] [CrossRef]
- Hempel, U.; Hefti, T.; Kalbacova, M.; Wolf-Brandstetter, C.; Dieter, P.; Schlottig, F. Response of osteoblast-like SAOS-2 cells to zirconia ceramics with different surface topographies. Clin. Oral Implants Res. 2010, 21, 174–181. [Google Scholar] [CrossRef]
- Hirano, T.; Sasaki, H.; Honma, S.; Furuya, Y.; Miura, T.; Yajima, Y.; Yoshinari, M. Proliferation and osteogenic differentiation of human mesenchymal stem cells on zirconia and titanium with different surface topography. Dent. Mater. J. 2015, 34, 872–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Sasaki, H.; Saito, K.; Honma, S.; Yajima, Y.; Yoshinari, M. Response of osteoblast-like cells to zirconia with different surface topography. Dent. Mater. J. 2013, 32, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.K.; Pati, F.; Mandal, D.; Dhara, S.; Biswas, K. In vitro evaluation of osteoconductivity and cellular response of zirconia and alumina based ceramics. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Faia-Torres, A.B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials 2014, 35, 9023–9032. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Kim, H.J.; Ko, J.S.; Choi, Y.S.; Ahn, M.W.; Kim, S.; Do, S.H. Comparative characteristics of porous bioceramics for an osteogenic response in vitro and in vivo. PLoS ONE 2013, 8, e84272. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ji, K.; Kirkham, J.; Yan, Y.; Boccaccini, A.R.; Kellett, M.; Jin, Y.; Yang, X.B. Bone tissue engineering by using a combination of polymer/Bioglass composites with human adipose-derived stem cells. Cell Tissue Res. 2014, 356, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Carinci, F.; Pezzetti, F.; Volinia, S.; Francioso, F.; Arcelli, D.; Farina, E.; Piattelli, A. Zirconium oxide: Analysis of MG63 osteoblast-like cell response by means of a microarray technology. Biomaterials 2004, 25, 215–228. [Google Scholar] [CrossRef]
- Altmann, B.; Rabel, K.; Kohal, R.J.; Proksch, S.; Tomakidi, P.; Adolfsson, E.; Bernsmann, F.; Palermo, P.; Fürderer, T.; Steinberg, T. Cellular transcriptional response to zirconia-based implant materials. Dent. Mater. 2017, 33, 241–255. [Google Scholar] [CrossRef]
- Cecchinato, F.; Karlsson, J.; Ferroni, L.; Gardin, C.; Galli, S.; Wennerberg, A.; Zavan, B.; Andeersson, M.; Jimbo, R. Osteogenic potential of human adipose-derived stromal cells on 3-dimensional mesoporous TiO2 coating with magnesium impregnation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.B.P.; Grenho, L.; Carvalho, A.; Fernandes, M.H.; Monteiro, F.J.; Cesar, P.F. Micropatterned Silica Films with Nanohydroxyapatite for Y-TZP Implants. J. Dent. Res. 2018, 97, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X. Receptor activator of nuclear factor-kappaB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review). Mol. Med. Rep. 2015, 11, 3212–3218. [Google Scholar] [CrossRef] [PubMed]




| Cells | Medium | Culture Formula |
|---|---|---|
| Human adipose-derived stromal cells (hADSC) | minimum essential medium–Alpha Eagle (α-MEM) (Lonza, Walkersville, MD, USA) | 10% fetal bovine serum, 1% amphotericin B (250 µg/mL), 1% glutamine (200 Mm), 1% penicillin (10,000 U/mL)/streptomycin (10,000 µg/mL) (Biochrom Merck, Berlin, Germany) |
| Primary human osteoblast (hOBs) | Primary human osteoblast (hOBs) | 12% fetal bovine serum, 1% amphotericin B (250 µg/mL), 1% glutamine (200 mM), 1% penicillin (10000 U/mL)/streptomycin (10,000 µg/mL) (Biochrom Merck, Germany). For osteogenic differentiation, 16 ng/mL dexamethasone (Merck Pharma, Darmstadt, Germany) was added to the medium. |
| Primary human gingiva fibroblasts (HGF) | DMEM medium (high glucose and L-glutamine; Gibco, USA) with ¼ Ham′s F12 nutrient mixtures (Sigma, Hamburg, Germany) | 10% fetal bovine serum, 1% amphotericin B (250 µg/mL), 1% penicillin (10,000 U/mL)/streptomycin (10,000 µg/mL) (all Biochrom Merck, Berlin, Germany) |
| Gene/Protein | Primer | Protein Assay/ELISA |
|---|---|---|
| Stem Cell Marker | ||
| ANPEP/CD13 | PPH05672A | |
| CD44 | PPH00114A | |
| THY1/CD90 | PPH02406G | |
| alkaline phosphatase | PPH01311F | |
| Osteogenic Marker | ||
| RUNX2 | PPH01897C | |
| TNFSF11/RANKL | PPH01048F | |
| osteoprotegerin | ab189580 | |
| osteopontin | ab192143 | |
| osteocalcin | ab195214 | |
| Housekeeping Genes | ||
| RPLP0 | PPH21138F | |
| B2M | PPH01094E | |
| GAPDH | PPH00150F | |
| HPRT1 | PPH01018C | |
| ACTB | PPH00073G | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Bohner, L.; Hanisch, M.; Kleinheinz, J.; Sielker, S. Influence of Implant Material and Surface on Differentiation and Proliferation of Human Adipose-Derived Stromal Cells. Int. J. Mol. Sci. 2018, 19, 4033. https://doi.org/10.3390/ijms19124033
Jung S, Bohner L, Hanisch M, Kleinheinz J, Sielker S. Influence of Implant Material and Surface on Differentiation and Proliferation of Human Adipose-Derived Stromal Cells. International Journal of Molecular Sciences. 2018; 19(12):4033. https://doi.org/10.3390/ijms19124033
Chicago/Turabian StyleJung, Susanne, Lauren Bohner, Marcel Hanisch, Johannes Kleinheinz, and Sonja Sielker. 2018. "Influence of Implant Material and Surface on Differentiation and Proliferation of Human Adipose-Derived Stromal Cells" International Journal of Molecular Sciences 19, no. 12: 4033. https://doi.org/10.3390/ijms19124033