Emerging Roles of Tumor Necrosis Factor-Stimulated Gene-6 in the Pathophysiology and Treatment of Atherosclerosis
Abstract
:1. Introduction
2. Structure of TSG-6
3. Expression and Regulation of TSG-6
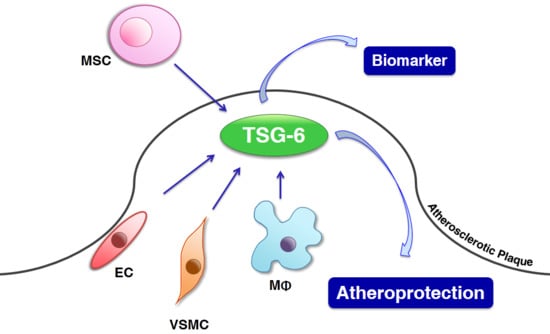
4. Roles of TSG-6
5. Effects of TSG-6 in ECs
6. Effects of TSG-6 in Monocytes/Macrophages
7. Effects of TSG-6 in VSMCs
8. Effects of TSG-6 on Atherosclerotic Lesion Development in ApoE-Deficient Mice
9. Roles of TSG-6 in Animal Atherosclerosis and Vascular Restenosis in Wire-Injury and Vein Graft Models
10. Expression of TSG-6 in Human Arteriosclerotic Lesions and Aneurysms
11. Potential Biomarker for CAD
12. Treatment of Atherosclerotic Diseases with TSG-6
13. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Research Ethics
Abbreviations
| CAD | Coronary artery disease |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| HUVEC | Human umbilical vein endothelial cell |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MACE | Major adverse cardiovascular event |
| MCP-1 | Monocyte chemotactic protein-1 |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem cell |
| NF-κB | Nuclear factor-κB |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-α |
| TSG-6 | Tumor necrosis factor-stimulated gene-6 |
| VSMC | Vascular smooth muscle cell |
References
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, K.; Kanome, T.; Matsuyama, T.; Koba, S.; Sakai, T.; Sato, K.; Hongo, S.; Nose, K.; Ota, H.; et al. Impact of salusin-α and -β on human macrophage foam cell formation and coronary atherosclerosis. Circulation 2008, 117, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Watanabe, T.; Iso, Y.; Koba, S.; Sakai, T.; Nagashima, M.; Arita, S.; Hongo, S.; Ota, H.; Kobayashi, Y.; et al. Preventive effects of heregulin-β1 on macrophage foam cell formation and atherosclerosis. Circ. Res. 2009, 105, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; et al. Counteractive effects of omentin-1 against atherogenesis. Cardiovasc. Res. 2016, 110, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Ozawa, N.; Mori, Y.; Takahashi, Y.; Watanabe-Kominato, K.; Shirai, R.; Watanabe, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; et al. Catestatin prevents macrophage-driven atherosclerosis but not arterial injury-induced neointimal hyperplasia. Thromb. Haemost. 2018, 118, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Sato, K.; Shirai, R.; Watanabe, R.; Yamamoto, K.; Watanabe, K.; Nohtomi, K.; Hirano, T.; Watanabe, T. Vasoprotective effects of urocortin 1 against atherosclerosis in vitro and in vivo. PLoS ONE 2014, 9, e110866. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Tajima, Y.; Hasegawa, A.; Takahashi, Y.; Kojima, M.; Watanabe, R.; Sato, K.; Shichiri, M.; Watanabe, T. Contrasting effects of stanniocalcin-related polypeptides on macrophage foam cell formation and vascular smooth muscle cell migration. Peptides 2016, 82, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Watanabe, T.; Terasaki, M.; Tomoyasu, M.; Nohtomi, K.; Kim-Kaneyama, J.; Miyazaki, A.; Hirano, T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Watanabe, H.; Takahashi, Y.; Kojima, M.; Konii, H.; Watanabe, K.; Shirai, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; et al. Atheroprotective effects of tumor necrosis factor-stimulated gene-6. JACC Basic Transl. Sci. 2016, 6, 494–509. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, G.W.; Ziff, E.B.; Vilcek, J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol. Cell. Biol. 1990, 10, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.G.; Maier, R.; Lotz, M.; Lee, S.; Klampfer, L.; Lee, T.H.; Vilcek, J. TSG-6: A TNF-, IL-1-, and LPS-inducible secreted glycoprotein associated with arthritis. J. Immunol. 1993, 151, 6593–6601. [Google Scholar] [PubMed]
- Fujimoto, T.; Savani, R.C.; Watari, M.; Day, A.J.; Strauss, J.F., 3rd. Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-α and prostaglandin E2. Am. J. Pathol. 2002, 160, 1495–1502. [Google Scholar] [CrossRef]
- Maina, V.; Cotena, A.; Doni, A.; Nebuloni, M.; Pasqualini, F.; Milner, C.M.; Day, A.J.; Mantovani, A.; Garlanda, C. Coregulation in human leukocytes of the long pentraxin PTX3 and TSG-6. J. Leukoc. Biol. 2009, 86, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Yamamoto, C.; Feng, Y.; Potter-Perigo, S.; Briggs, W.H.; Landschulz, K.T.; Turi, T.G.; Thompson, J.F.; Libby, P.; Wight, T.N. Mechanical strain induces specific changes in the synthesis and organization of proteoglycans by vascular smooth muscle cells. J. Biol. Chem. 2001, 276, 13847–13851. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Li, L.; Chen, Y.; Luo, A.; Ni, Z.; Liu, J.; Yuan, Y.; Shi, M.; Chen, B.; Long, D.; et al. Mesenchymal stem cells ameliorated glucolipotoxicity in HUVECs through TSG-6. Int. J. Mol. Sci. 2016, 17, 483. [Google Scholar] [CrossRef] [PubMed]
- Nagyeri, G.; Radacs, M.; Ghassemi-Nejad, T.; Tryniszewska, B.; Olasz, K.; Hutas, G.; Gyorfy, Z.; Hacall, V.C.; Glant, T.; Mikecz, K. TSG-6 protein, a negative regulator of inflammatory arthritis, forms a ternary complex with murine mast cell tryptases and heparin. J. Boil. Chem. 2011, 286, 23559–23569. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Wisniewski, H.G.; Vilcek, J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J. Cell Biol. 1992, 116, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chan, C.K.; Braun, K.R.; Green, P.S.; O’Brien, K.D.; Chait, A.; Day, A.J.; Wight, T.N. Monocyte-to-macrophage differentiation: Synthesis and secretion of a complex extracellular matrix. J. Biol. Chem. 2012, 287, 14122–14135. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, M.T.; Howat, S.L.; Dudhia, J.; Murphy, J.M.; Barry, F.P.; Edwards, J.C.; Day, A.J. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthr. Cartil. 2001, 9, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.G.; Vilcek, J. TSG-6: An IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997, 8, 143–156. [Google Scholar] [CrossRef]
- Milner, C.M.; Higman, V.A.; Day, A.J. TSG-6: A pluripotent inflammatory mediator? Biochem. Soc. Trans. 2006, 34, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Choi, H.; Nishida, H.; Oh, J.Y.; Gregory, C.; Lee, R.H.; Yu, J.M.; Watanabe, J.; An, S.Y.; Bartosh, T.J.; et al. Scalable production of a multifunctional protein (TSG-6) that aggregates with itself and the CHO cells that synthesize it. PLoS ONE 2016, 11, e0147553. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.E.; Cheng, G.; Swaidani, S.; Aronica, M.A.; Weigel, P.H.; Hascall, V.C. Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J. Biol. Chem. 2013, 288, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, D.J.; Mulloy, B.; Forster, M.J.; Blundell, C.D.; Fries, E.; Milner, C.M.; Day, A.J. Characterization of the interaction between tumor necrosis factor-stimulated gene-6 and heparin: Implications for the inhibition of plasmin in extracellular matrix microenvironments. J. Biol. Chem. 2005, 280, 27044–27055. [Google Scholar] [CrossRef] [PubMed]
- Leali, D.; Inforzato, A.; Ronca, R.; Bianchi, R.; Belleri, M.; Coltrini, D.; Di Salle, E.; Sironi, M.; Norata, G.D.; Bottazzi, B.; et al. Long pentraxin 3/tumor necrosis factor-stimulated gene-6 interaction: A biological rheostat for fibroblast growth factor 2-mediated angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bardos, T.; Kamath, R.V.; Mikecz, K.; Glant, T.T. Anti-inflammatory and chondroprotective effect of TGF-6 (tumor necrosis factor-α stimulated gene-6) in murine models of experimental arthritis. Am. J. Pathol. 2001, 159, 1711–1721. [Google Scholar] [CrossRef]
- Oh, J.Y.; Roddy, G.W.; Choi, H.; Lee, R.H.; Ylöstalo, J.H.; Rosa, R.H., Jr.; Prockop, D.J. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. USA 2010, 107, 16875–16880. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.M.; Zhao, S.; Zhou, L.L.; Fang, X.S.; Fu, Y.; Huang, Z.T. Mesenchymal stem cells transplantation suppresses inflammatory responses in global cerebral ischemia: Contribution of TNF-α-induced protein 6. Acta Pharmacol. Sin. 2013, 34, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Szántó, S.; Bárdos, T.; Gál, I.; Glant, T.T.; Mikecz, K. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 2004, 50, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Mindrescu, C.; Dias, A.A.; Olszewski, R.J.; Klein, M.J.; Reis, L.F.; Wisniewski, H.G. Reduced susceptibility to collagen-induced arthritis in DBA/1J mice expressing the TSG-6 transgene. Arthritis Rheum. 2002, 46, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Mindrescu, C.; Thorbecke, G.J.; Klein, M.J.; Vilcek, J.; Wisniewski, H.G. Amelioration of collagen-induced arthritis in DBA/1J mice by recombinant TSG-6, a tumor necrosis factor/interleukin-1-inducible protein. Arthritis Rheum. 2000, 43, 2668–2677. [Google Scholar] [CrossRef]
- Watanabe, J.; Shetty, A.K.; Hattiangady, B.; Kim, D.K.; Foraker, J.E.; Nishida, H.; Prockop, D.J. Administration of TSG-6 improves memory after traumatic brain injury in mice. Neurobiol. Dis. 2013, 59C, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Li, Q.; Ryu, M.O.; Ahn, J.O.; Bhang, D.H.; Jung, Y.C.; Youn, H.Y. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Sci. Rep. 2017, 7, 5187. [Google Scholar] [CrossRef] [PubMed]
- Carelli, S.; Colli, M.; Vinci, V.; Caviggioli, F.; Klinger, M.; Gorio, A. Mechanical activation of adipose tissue and derived mesenchymal stem cells: Novel anti-inflammatory properties. Int. J. Mol. Sci. 2018, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Song, H.B.; Park, S.Y.; Ko, J.H.; Park, J.W.; Yoon, C.H.; Kim, D.H.; Kim, J.H.; Kim, M.K.; Lee, R.H.; Prockop, D.J.; et al. Mesenchymal stromal cells inhibit inflammatory lymphangiogenesis in the cornea by suppressing macrophage in a TSG-6-dependent manner. Mol. Ther. 2018, 26, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Ichiseki, T.; Shimazaki, M.; Ueda, Y.; Ueda, S.; Tsuchiya, M.; Souma, D.; Kaneuji, A.; Kawahara, N. Intraarticularly-injected mesenchymal stem cells stimulate anti-inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate-induced rat arthritis model. Int. J. Mol. Sci. 2018, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.V.; La, M.; Getting, S.J.; Day, A.J.; Perretti, M. Inhibitory effects of TSG-6 link module on leukocyte-endothelial cell interactions in vitro and in vivo. Microcirculation 2004, 11, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.; Day, A.J.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Mindrescu, C.; Le, J.; Wisniewski, H.G.; Vilcek, J. Up-regulation of cyclooxygenase-2 expression by TSG-6 protein in macrophage cell line. Biochem. Biophys. Res. Commun. 2005, 330, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, B.; Wang, H.; Tao, Q.; Ge, S.; Zhai, Z. Tumor necrosis factor alpha-stimulated gene-6 (TSG-6) inhibits the inflammatory response by inhibiting the activation of P38 and JNK signaling pathway and decreases the restenosis of vein grafts in rats. Heart Vessels 2017, 32, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mora, R.; Akhayani, N.; Haudenschild, C.C.; Liau, G. Growth factor and cytokine-regulated hyaluronan-binding protein TSG-6 is localized to the injury-induced rat neointima and confers enhanced growth in vascular smooth muscle cells. Circ. Res. 1997, 81, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Hu, S.W.; Zhang, Q.H.; Xia, A.X.; Jiang, Z.X.; Chen, X.M. Mesenchymal stem cells stabilize atherosclerotic vulnerable plaque by anti-inflammatory properties. PLoS ONE 2015, 10, e0136026. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Xie, J.; Green, L.A.; McCready, R.A.; Motaganahalli, R.L.; Fajardo, A.; Babbey, C.C.; Murphy, M.P. TSG-6 is highly expressed in human abdominal aortic aneurysms. J. Surg. Res. 2017, 220, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kota, D.J.; Wiggins, L.L.; Yoon, N.; Lee, R.H. TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes 2013, 62, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shao, Y.; Mei, Y.; Zhang, L.; Li, Q.; Li, D.; Chen, X. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: Accumulating in the lung and secreting tumor necrosis factor α-stimulating gene-6. Stem Cell Res. Ther. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, Q.; Zhang, L.; Lin, H.; Hu, J.; Li, D.; Chen, X. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS ONE 2012, 7, e43768. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Shoval, Y.; Barhum, Y.; Sadan, O.; Yust-Katz, S.; Ben-Zur, T.; Lev, N.; Offen, D. Transplantation of placenta-derived mesenchymal stem cells in the EAE mouse model of MS. J. Mol. Neurosci. 2012, 48, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Conejos-Sánchez, I.; Griffin, B.T.; O’Driscoll, C.M.; Alonso, M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 337–354. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, R.; Sato, Y.; Ozawa, N.; Takahashi, Y.; Koba, S.; Watanabe, T. Emerging Roles of Tumor Necrosis Factor-Stimulated Gene-6 in the Pathophysiology and Treatment of Atherosclerosis. Int. J. Mol. Sci. 2018, 19, 465. https://doi.org/10.3390/ijms19020465
Watanabe R, Sato Y, Ozawa N, Takahashi Y, Koba S, Watanabe T. Emerging Roles of Tumor Necrosis Factor-Stimulated Gene-6 in the Pathophysiology and Treatment of Atherosclerosis. International Journal of Molecular Sciences. 2018; 19(2):465. https://doi.org/10.3390/ijms19020465
Chicago/Turabian StyleWatanabe, Rena, Yuki Sato, Nana Ozawa, Yui Takahashi, Shinji Koba, and Takuya Watanabe. 2018. "Emerging Roles of Tumor Necrosis Factor-Stimulated Gene-6 in the Pathophysiology and Treatment of Atherosclerosis" International Journal of Molecular Sciences 19, no. 2: 465. https://doi.org/10.3390/ijms19020465
APA StyleWatanabe, R., Sato, Y., Ozawa, N., Takahashi, Y., Koba, S., & Watanabe, T. (2018). Emerging Roles of Tumor Necrosis Factor-Stimulated Gene-6 in the Pathophysiology and Treatment of Atherosclerosis. International Journal of Molecular Sciences, 19(2), 465. https://doi.org/10.3390/ijms19020465