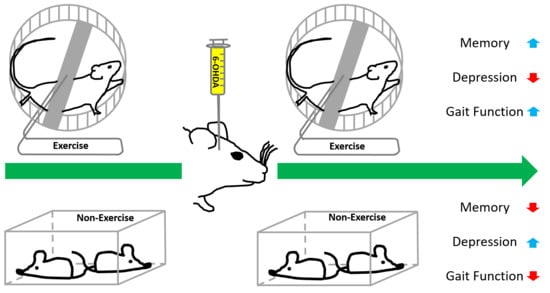
Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
2.1. Behavioral Amelioration in Parkinson’s Disease Model of Rats Receiving Exercise-Novel Object Recognition (NOR), Rotational Test (RT) and Forced Swimming Test (FST)
2.2. Spatiotemporal Gait Analysis
2.3. TH Immunohistochemistry
2.4. Upregulation of BMX (Bone Marrow Tyrosine Kinase in Chromosome X), Brain-Derived Neurotrophic Factor (BDNF), and TH (Tyrosine Hydroxylase) Expression in the Striatum of Rats Receiving Exercise
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Exercise
4.3. Chronic Hemi-Parkinsonian Rat Model
4.4. Novel Object Recognition (NOR)
4.5. Spatiotemporal Analysis of Gait Patterns
4.6. Forced Swimming Test (FST)
4.7. Fixation and Sectioning
4.8. Immunohistochemistry and Immunofluorescence
4.9. Immunoblotting
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| 6-OHDA | 6-Hydroxydopamine |
| TH | Tyrosine Hydroxylase |
| DA | Dopaminergic |
| SN | Substantia Nigra |
References
- Sethi, K.D. Clinical aspects of Parkinson disease. Curr. Opin. Neurol. 2002, 15, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Treves, T.A.; Simon, E.S.; Shabtai, H.; Orlov, Y.; Kandinov, B.; Paleacu, D.; Korczyn, A.D. Freezing of gait in patients with advanced Parkinson’s disease. J. Neural Transm. 2001, 108, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chee, R.; Murphy, A.; Danoudis, M.; Georgiou-Karistianis, N.; Iansek, R. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain 2009, 132 Pt 8, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Dom, R.; De Weerdt, W.; Desloovere, K.; Fieuws, S.; Broens-Kaucsik, E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov. Disord. 2001, 16, 1066–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blin, O.; Ferrandez, A.M.; Serratrice, G. Quantitative analysis of gait in Parkinson patients: Increased variability of stride length. J. Neurol. Sci. 1990, 98, 91–97. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Chen, J.J.; Chen, L.H.; Chiang, P.T.; Lee, H.Y. Time-course gait analysis of hemiparkinsonian rats following 6-hydroxydopamine lesion. Behav. Brain Res. 2011, 222, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, H.A.; Koivula, P.P.; Necarsulmer, J.C.; Whitaker, K.W.; Harvey, B.K. Step Sequence Is a Critical Gait Parameter of Unilateral 6-OHDA Parkinson’s Rat Models. Cell Transplant. 2017, 26, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hsieh, T.H.; Liang, J.I.; Yeh, M.L.; Chen, J.J. Quantitative video-based gait pattern analysis for hemiparkinsonian rats. Med. Biol. Eng. Comput. 2012, 50, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, M.J.; Smeyne, R.J. Exercise: Is it a neuroprotective and if so, how does it work? Parkinsonism Relat. Disord. 2014, 20 (Suppl. 1), S123–S127. [Google Scholar] [CrossRef]
- Tajiri, N.; Yasuhara, T.; Shingo, T.; Kondo, A.; Yuan, W.; Kadota, T.; Wang, F.; Baba, T.; Tayra, J.T.; Morimoto, T.; et al. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010, 1310, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.A.; Koo, B.S.; An, G.J.; Jeon, S. Effects of Treadmill Exercise on the Recovery of Dopaminergic Neuron Loss and Muscle Atrophy in the 6-OHDA Lesioned Parkinson’s Disease Rat Model. Korean J. Physiol. Pharmacol. 2012, 16, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ravina, B.; Camicioli, R.; Como, P.G.; Marsh, L.; Jankovic, J.; Weintraub, D.; Elm, J. The impact of depressive symptoms in early Parkinson disease. Neurology 2007, 69, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Damian, J. Parkinson disease: Depression and anxiety in Parkinson disease. Nat. Rev. Neurol. 2010, 6, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Litvan, I. Mild cognitive impairment in Parkinson’s disease. Minerva Med. 2011, 102, 441–459. [Google Scholar] [PubMed]
- Smith, L.M.; Schiess, M.C.; Coffey, M.P.; Klaver, A.C.; Loeffler, D.A. α-Synuclein and anti-α-synuclein antibodies in Parkinson’s disease, atypical Parkinson syndromes, REM sleep behavior disorder, and healthy controls. PLoS ONE 2012, 7, e52285. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Agid, Y.; Barone, P.; Jenner, P.; Lemke, M.R.; Poewe, W.; Rascol, O.; Reichmann, H.; Tolosa, E. Perspectives on recent advances in the understanding and treatment of Parkinson’s disease. Eur. J. Neurol. 2009, 16, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C. Future drug targets for Parkinson’s disease. Bull. Acad. Natl. Med. 2012, 196, 1369–1377, discussion 1377–1399. [Google Scholar] [PubMed]
- Gorton, L.M.; Vuckovic, M.G.; Vertelkina, N.; Petzinger, G.M.; Jakowec, M.W.; Wood, R.I. Exercise effects on motor and affective behavior and catecholamine neurochemistry in the MPTP-lesioned mouse. Behav. Brain Res. 2010, 213, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S., Jr.; Araujo, A.L.; da-Cunha, T.R.; Speck, A.E.; Ignacio, Z.M.; De-Mello, N.; Prediger, R.D. Physical exercise improves motor and short-term social memory deficits in reserpinized rats. Brain Res. Bull. 2009, 79, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Goes, A.T.; Souza, L.C.; Filho, C.B.; Del Fabbro, L.; De Gomes, M.G.; Boeira, S.P.; Jesse, C.R. Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience 2014, 256, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Cruise, K.E.; Bucks, R.S.; Loftus, A.M.; Newton, R.U.; Pegoraro, R.; Thomas, M.G. Exercise and Parkinson’s: Benefits for cognition and quality of life. Acta Neurol. Scand. 2011, 123, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Zid, D.; Russell, J.; Malone, A.; Rendon, A.; Wehr, A.; Li, X. Effects of a formal exercise program on Parkinson’s disease: A pilot study using a delayed start design. Parkinsonism Relat. Disord. 2014, 20, 106–111. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.M.; Richard, I.H.; DeLong, M.R. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol. Psychiatry 2003, 54, 363–375. [Google Scholar] [CrossRef]
- Benito-Leon, J.; Louis, E.D.; Posada, I.J.; Sanchez-Ferro, A.; Trincado, R.; Villarejo, A.; Mitchell, A.J.; Bermejo-Pareja, F. Population-based case-control study of cognitive function in early Parkinson’s disease (NEDICES). J. Neurol. Sci. 2011, 310, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Dalaker, T.O.; Zivadinov, R.; Ramasamy, D.P.; Beyer, M.K.; Alves, G.; Bronnick, K.S.; Tysnes, O.B.; Aarsland, D.; Larsen, J.P. Ventricular enlargement and mild cognitive impairment in early Parkinson’s disease. Mov. Disord. 2011, 26, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Tuon, T.; Valvassori, S.S.; Lopes-Borges, J.; Luciano, T.; Trom, C.B.; Silva, L.A.; Quevedo, J.; Souza, C.T.; Lira, F.S.; Pinho, R.A. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 2012, 227, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Kim, T.W.; Lee, J.M.; Sung, Y.H.; Lim, B.V. Treadmill exercise alleviates depressive symptoms in rotenone-induced Parkinson disease rats. J. Exerc. Rehabil. 2017, 13, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef]
- Sawamoto, N.; Piccini, P.; Hotton, G.; Pavese, N.; Thielemans, K.; Brooks, D.J. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain 2008, 131 Pt 5, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Hariri, A.R.; Fera, F.; Smith, W.G.; Chase, T.N.; Hyde, T.M.; Weinberger, D.R.; Mattay, V.S. Dopamine modulates the response of the human amygdala: A study in Parkinson’s disease. J. Neurosci. 2002, 22, 9099–9103. [Google Scholar] [PubMed]
- Tuon, T.; Valvassori, S.S.; Dal Pont, G.C.; Paganini, C.S.; Pozzi, B.G.; Luciano, T.F.; Souza, P.S.; Quevedo, J.; Souza, C.T.; Pinho, R.A. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res. Bull. 2014, 108, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S., Jr.; Castro, A.A.; Moreira, E.L.; Glaser, V.; Santos, A.R.; Tasca, C.I.; Latini, A.; Prediger, R.D. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 2011, 132, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Robinson, D.; Pretlow, T.G.; Kung, H.J. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3644–3649. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Kung, H.J. Signaling network of the Btk family kinases. Oncogene 2000, 19, 5651–5661. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Huang, C.L.; Kung, H.J.; Huang, C.Y. Proteolytic activation of ETK/Bmx tyrosine kinase by caspases. J. Biol. Chem. 2001, 276, 17672–17678. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Huang, L.M.; Kung, H.J.; Ann, D.K.; Shih, H.M. The role of tyrosine kinase Etk/Bmx in EGF-induced apoptosis of MDA-MB-468 breast cancer cells. Oncogene 2004, 23, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Wu, C.C.; Chang, C.F.; Chen, Y.H.; Chiu, W.T.; Lou, Y.H.; Chen, Y.H.; Shih, H.M.; Chiang, Y.H. Suppression of Etk/Bmx protects against ischemic brain injury. Cell Transplant. 2012, 21, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Aggleton, J.P. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav. Brain Res. 1997, 88, 181–193. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, S.-C.; Chen, K.-Y.; Lai, J.-H.; Wu, C.-C.; Yu, Y.-W.; Luo, Y.; Hsieh, T.-H.; Chiang, Y.-H. Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 508. https://doi.org/10.3390/ijms19020508
Hsueh S-C, Chen K-Y, Lai J-H, Wu C-C, Yu Y-W, Luo Y, Hsieh T-H, Chiang Y-H. Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2018; 19(2):508. https://doi.org/10.3390/ijms19020508
Chicago/Turabian StyleHsueh, Shih-Chang, Kai-Yun Chen, Jing-Huei Lai, Chung-Che Wu, Yu-Wen Yu, Yu Luo, Tsung-Hsun Hsieh, and Yung-Hsiao Chiang. 2018. "Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease" International Journal of Molecular Sciences 19, no. 2: 508. https://doi.org/10.3390/ijms19020508
APA StyleHsueh, S.-C., Chen, K.-Y., Lai, J.-H., Wu, C.-C., Yu, Y.-W., Luo, Y., Hsieh, T.-H., & Chiang, Y.-H. (2018). Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences, 19(2), 508. https://doi.org/10.3390/ijms19020508