Deficiency of Invariant Natural Killer T Cells Does Not Protect Against Obesity but Exacerbates Atherosclerosis in Ldlr−/− Mice
Abstract
:1. Introduction
2. Results
2.1. Natural Killer T (NKT) Cell Deficiency Is Associated with Weight Gain in Ldlr−/− Mice on a High Fat, High Sucrose Cholesterol Enriched (HFHSC) Diet
2.2. Obesity-Induced Metabolic Abnormalities Are Observed in the Presence and Absence of NKT Cells
2.3. Adipose Tissue Inflammation Is Worsened in Obese Ja18−/−Ldlr−/− Mice Devoid of iNKT Cells
2.4. Hepatic Steatosis Is Observed in Obese Mice in the Presence and Absence of iNKT Cells
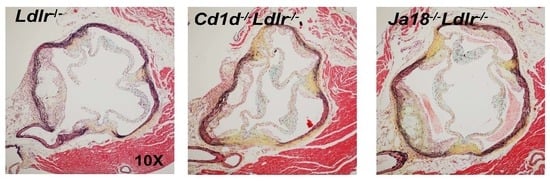
2.5. Aortic Atherosclerosis Is Increased in Ja18−/−Ldlr−/− Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Analytical Procedures
4.3. Real-Time Quantitative PCR
4.4. Histology, Immunohistochemistry, and Atherosclerosis Quantification
4.5. Statistics
Acknowledgments
Authors Contribution
Conflicts of Interest
Abbreviations
| FPLC | fast performance liquid chromatography |
| HFHSC | high fat, high sucrose, cholesterol |
| iNKT | invariant Natural Killer T |
| Ldlr | Low density lipoprotein receptor |
| SAA | serum amyloid A |
| TCR | T cell receptor |
| Treg | T regulatory |
References
- Burzyn, D.; Benoist, C.; Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 2013, 14, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013, 17, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: Key players in immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bonamichi, B.; Lee, J. Unusual suspects in the development of obesity-induced inflammation and insulin resistance: NK cells, iNKT cells, and ILCs. Diabetes Metab. J. 2017, 41, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 2013, 34, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Rossjohn, J.; Pellicci, D.G.; Patel, O.; Gapin, L.; Godfrey, D.I. Recognition of CD1d-restricted antigens by natural killer t cells. Nat. Rev. Immunol. 2012, 12, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Kim, J.I.; Park, Y.J.; Hwang, I.J.; Lee, Y.S.; Sohn, J.H.; Lee, S.K.; Alfadda, A.A.; Kim, S.S.; Choi, S.H.; et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol. Cell. Biol. 2013, 33, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Hoshino, M.; Fujita, K.; Iizuka, M.; Fujii, S.; Clingan, C.S.; van Kaer, L.; Iwabuchi, K. Adipocyte-specific Cd1d-deficiency mitigates diet-induced obesity and insulin resistance in mice. Sci. Rep. 2016, 6, 28473. [Google Scholar] [CrossRef] [PubMed]
- Kain, L.; Costanzo, A.; Webb, B.; Holt, M.; Bendelac, A.; Savage, P.B.; Teyton, L. Endogenous ligands of natural killer T cells are alpha-linked glycosylceramides. Mol. Immunol. 2015, 68, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Tatituri, R.V.; Heiss, C.; Watts, G.F.; Hsu, F.F.; Veerapen, N.; Cox, L.R.; Azadi, P.; Besra, G.S.; Brenner, M.B. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc. Natl. Acad. Sci. USA 2014, 111, 13433–13438. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.J.; Tatituri, R.V.; Almeida, C.F.; Le Nours, J.; Bhowruth, V.; Johnson, D.; Uldrich, A.P.; Hsu, F.F.; Brigl, M.; Besra, G.S.; et al. Identification of a potent microbial lipid antigen for diverse NKT cells. J. Immunol. 2015, 195, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.; Nowak, M.; Varghese, B.; Clark, J.; Hogan, A.E.; Toxavidis, V.; Balk, S.P.; O’Shea, D.; O’Farrelly, C.; Exley, M.A. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012, 37, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Turner, M.S.; Ding, Y.; Goodspeed, L.; Wang, S.; Buckner, J.H.; O’Brien, K.; Getz, G.S.; Reardon, C.A.; Chait, A. Increased levels of invariant natural killer T lymphocytes worsen metabolic abnormalities and atherosclerosis in obese mice. J. Lipid Res. 2013, 54, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.; O’Shea, D.; Winter, D.C.; Geoghegan, J.; Doherty, D.G.; O’Farrelly, C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 2009, 39, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Parekh, V.V.; Gabriel, C.L.; Bracy, D.P.; Marks-Shulman, P.A.; Tamboli, R.A.; Kim, S.; Mendez-Fernandez, Y.V.; Besra, G.S.; Lomenick, J.P.; et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl. Acad. Sci. USA 2012, 109, E1143–E1152. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.; Michelet, X.; Zhang, S.; Brennan, P.J.; Moseman, A.; Lester, C.; Besra, G.; Vomhof-Dekrey, E.E.; Tighe, M.; Koay, H.F.; et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat. Immunol. 2015, 16, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sag, D.; Krause, P.; Hedrick, C.C.; Kronenberg, M.; Wingender, G. IL-10-producing NKT10 cells are a distinct regulatory invariant nkt cell subset. J. Clin. Investig. 2014, 124, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Lee, H.Y.; Gillum, M.P.; Annicelli, C.; Guigni, B.A.; Shulman, G.I.; Medzhitov, R. Impact of CD1d deficiency on metabolism. PLoS ONE 2011, 6, e25478. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.S.; Rakhshandehroo, M.; van de Graaf, S.F.; Venken, K.; Koppen, A.; Stienstra, R.; Prop, S.; Meerding, J.; Hamers, N.; Besra, G.; et al. Natural killer T cells in adipose tissue prevent insulin resistance. J. Clin. Investig. 2012, 122, 3343–3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, M.; Andoh, Y.; Clingan, C.S.; Ogura, H.; Fujii, S.; Eshima, K.; Nakayama, T.; Taniguchi, M.; Hirata, N.; Ishimori, N.; et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS ONE 2012, 7, e30568. [Google Scholar] [CrossRef] [PubMed]
- Strodthoff, D.; Lundberg, A.M.; Agardh, H.E.; Ketelhuth, D.F.; Paulsson-Berne, G.; Arner, P.; Hansson, G.K.; Gerdes, N. Lack of invariant natural killer T cells affects lipid metabolism in adipose tissue of diet-induced obese mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Mantell, B.S.; Stefanovic-Racic, M.; Yang, X.; Dedousis, N.; Sipula, I.J.; O’Doherty, R.M. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS ONE 2011, 6, e19831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Murphy, B.V.; You, Q.; Wang, H.; de La Houssaye, B.A.; Reilly, T.P.; Friedman, J.E.; Ju, C. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS ONE 2014, 9, e80949. [Google Scholar] [CrossRef] [PubMed]
- Exley, M.A.; Hand, L.; O’Shea, D.; Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 2014, 223, R41–R48. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Han, C.Y.; Chiba, T.; McMillen, T.S.; Wang, S.A.; Haw, A., 3rd; Kirk, E.A.; O’Brien, K.D.; Chait, A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 685–691. [Google Scholar] [CrossRef] [PubMed]
- VanderLaan, P.A.; Reardon, C.A.; Sagiv, Y.; Blachowicz, L.; Lukens, J.; Nissenbaum, M.; Wang, C.R.; Getz, G.S. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am. J. Pathol. 2007, 170, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Major, A.S.; Wilson, M.T.; McCaleb, J.L.; Su, Y.R.; Stanic, A.K.; Joyce, S.; Van Kaer, L.; Fazio, S.; Linton, M.F. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Tupin, E.; Nicoletti, A.; Elhage, R.; Rudling, M.; Ljunggren, H.G.; Hansson, G.K.; Berne, G.P. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 2004, 199, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Iwabuchi, K.; Fujii, S.; Ishimori, N.; Dashtsoodol, N.; Watano, K.; Mishima, T.; Iwabuchi, C.; Tanaka, S.; Bezbradica, J.S.; et al. Natural killer T cells accelerate atherogenesis in mice. Blood 2004, 104, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, A.M.; Chapman, H.A.; Charo, I.F. Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Burchat, S.; Gage, J.; Hasu, M.; Thabet, M.; Willcox, L.; Ramsamy, T.A.; Whitman, S.C. Deficiency of invariant Valpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc. Res. 2008, 78, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Deiuliis, J.; Shah, Z.; Shah, N.; Needleman, B.; Mikami, D.; Narula, V.; Perry, K.; Hazey, J.; Kampfrath, T.; Kollengode, M.; et al. Visceral adipose inflammation in obesity is associated with critical alterations in Tregulatory cell numbers. PLoS ONE 2011, 6, e16376. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Zhao, M.; Budelsky, A.; de Mingo Pulido, A.; Day, J.; Fu, Z.; Siegel, L.; Smith, D.; Kronenberg, M. A new mouse strain for the analysis of invariant NKT cell function. Nat. Immunol. 2015, 16, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Backhed, F. Assessing the human gut microbiota in metabolic diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, E.E.; Matsumoto, T.; Lindenbergh, D.; Willemsen, R.; Kaser, A.; Simons-Oosterhuis, Y.; Brugman, S.; Yamaguchi, K.; Ishikawa, H.; Aiba, Y.; et al. CD1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Investig. 2009, 119, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Ohman, M.K.; Luo, W.; Wang, H.; Guo, C.; Abdallah, W.; Russo, H.M.; Eitzman, D.T. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 2011, 219, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.K.; Zhou, Z.; Zhang, J.; Zeng, R.; Wu, J.; Eitzman, D.T.; Chen, Y.E.; Chang, L. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Lewis, K.E.; Kirk, E.A.; McDonald, T.O.; Wang, S.; Wight, T.N.; O’Brien, K.D.; Chait, A. Increase in serum amyloid A evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation 2004, 110, 540–545. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian, S.; Goodspeed, L.; Wang, S.; Ding, Y.; O’Brien, K.D.; Getz, G.S.; Chait, A.; Reardon, C.A. Deficiency of Invariant Natural Killer T Cells Does Not Protect Against Obesity but Exacerbates Atherosclerosis in Ldlr−/− Mice. Int. J. Mol. Sci. 2018, 19, 510. https://doi.org/10.3390/ijms19020510
Subramanian S, Goodspeed L, Wang S, Ding Y, O’Brien KD, Getz GS, Chait A, Reardon CA. Deficiency of Invariant Natural Killer T Cells Does Not Protect Against Obesity but Exacerbates Atherosclerosis in Ldlr−/− Mice. International Journal of Molecular Sciences. 2018; 19(2):510. https://doi.org/10.3390/ijms19020510
Chicago/Turabian StyleSubramanian, Savitha, Leela Goodspeed, Shari Wang, Yilei Ding, Kevin D. O’Brien, Godfrey S. Getz, Alan Chait, and Catherine A. Reardon. 2018. "Deficiency of Invariant Natural Killer T Cells Does Not Protect Against Obesity but Exacerbates Atherosclerosis in Ldlr−/− Mice" International Journal of Molecular Sciences 19, no. 2: 510. https://doi.org/10.3390/ijms19020510
APA StyleSubramanian, S., Goodspeed, L., Wang, S., Ding, Y., O’Brien, K. D., Getz, G. S., Chait, A., & Reardon, C. A. (2018). Deficiency of Invariant Natural Killer T Cells Does Not Protect Against Obesity but Exacerbates Atherosclerosis in Ldlr−/− Mice. International Journal of Molecular Sciences, 19(2), 510. https://doi.org/10.3390/ijms19020510