Preparation of Fish Skin Gelatin-Based Nanofibers Incorporating Cinnamaldehyde by Solution Blow Spinning
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Fiber-Forming Emulsions (FFEs)
2.1.1. Particle Size and Particle Size Distribution
2.1.2. Viscosity
2.1.3. Surface Tension
2.2. Characterization of Nanofibers
2.2.1. Morphology and Diameter
2.2.2. Cinnamaldehyde Distribution within Nanofibers
2.3. Antimicrobial Activity and Storage Stability of Nanofibers
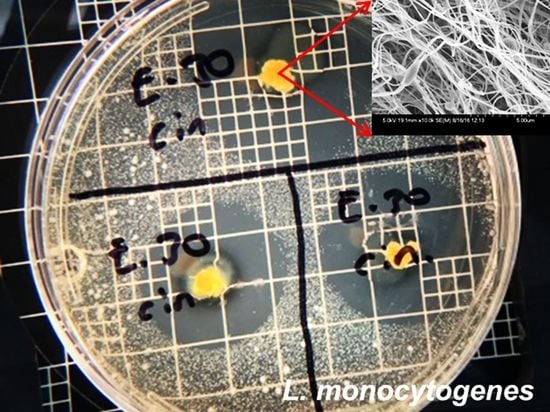
2.3.1. Antimicrobial Activity
2.3.2. Storage Stability
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fiber-Forming Emulsions (FFEs)
3.3. Characterization of Fiber-Forming Emulsions (FFEs)
3.3.1. Particle Size and Particle Size Distribution
3.3.2. Viscosity
3.3.3. Surface Tension
3.4. Preparation of Nanofibers
3.5. Characterization of Nanofibers
3.5.1. Scanning Electron Microscopy (SEM)
3.5.2. Transmission Electron Microscopy (TEM)
3.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.6. Antimicrobial Activity and Storage Stability of Nanofibers
3.6.1. Antimicrobial Activity
3.6.2. Storage Stability
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FSG | fish skin gelatin |
| FFE | fiber-forming emulsion |
| SBS | solution blow spinning |
| FDA | Food and Drug Administration |
| GRAS | Generally Recognized as Safe |
| PDI | polydispersity index |
| RH | relative humidity |
| SEM | scanning electron microscopy |
| AFD | average fiber diameter |
| TEM | transmission electron microscopy |
| FTIR | fourier transform infrared spectroscopy |
| CFU | colony-forming-units |
| FID | flame ionization detector |
| RRF | relative response factor |
| ANOVA | analysis of variance |
References
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of β-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Makwana, S.; Choudhary, R.; Dogra, N.; Kohli, P.; Haddock, J. Nanoencapsulation and immobilization of cinnamaldehyde for developing antimicrobial food packaging material. LWT Food Sci. Technol. 2014, 57, 470–476. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Storage stability and antibacterial activity against Escherichia coli O157: H7 of carvacrol in edible apple films made by two different casting methods. J. Agric. Food Chem. 2008, 56, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Sanchez, C.; Batlle, R.; Nerin, C. Solid-and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; de Jesús Ornelas-Paz, J.; del Toro-Sánchez, C.L.; Márquez-Ríos, E.; Silva-Beltrán, N.P.; Cira-Chávez, L.A.; Burruel-Ibarra, S.E. Mechanical, barrier and antioxidant properties of chitosan films incorporating cinnamaldehyde. J. Polym. Environ. 2017, 1–10. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT Food Sci. Technol. 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Blanco-Padilla, A.; López-Rubio, A.; Loarca-Piña, G.; Gómez-Mascaraque, L.G.; Mendoza, S. Characterization, release and antioxidant activity of curcumin-loaded amaranth-pullulan electrospun fibers. LWT Food Sci. Technol. 2015, 63, 1137–1144. [Google Scholar] [CrossRef]
- Neo, Y.P.; Swift, S.; Ray, S.; Gizdavic-Nikolaidis, M.; Jin, J.; Perera, C.O. Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem. 2013, 141, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced thermal stability of eugenol by cyclodextrin inclusion complex encapsulated in electrospun polymeric nanofibers. J. Agric. Food Chem. 2013, 61, 8156–8165. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Uyar, T. Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef]
- Jiang, S.; Lv, J.; Ding, M.; Li, Y.; Wang, H.; Jiang, S. Release behavior of tetracycline hydrochloride loaded chitosan/poly(lactic acid) antimicrobial nanofibrous membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.M.; Casey, B.J.; Sikorski, M.J.; Wu, K.L.; Tutak, W.; Sandler, A.D.; Kofinas, P. In situ deposition of plga nanofibers via solution blow spinning. ACS Macro Lett. 2014, 3, 249–254. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.-S.; Valenzuela-Medina, D.; Du, W.-X.; Gregorski, K.S.; Williams, T.G.; Wood, D.F.; Glenn, G.M.; Orts, W.J. Solution blow spun poly(lactic acid)/hydroxypropyl methylcellulose nanofibers with antimicrobial properties. Eur. Polym. J. 2014, 54, 1–10. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A review of the fundamental principles and applications of solution blow spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution blow spinning: A new method to produce micro- and nanofibers from polymer solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Moraes, E.A.; Costa, R.G.F.; Afonso, A.S.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Nano and submicrometric fibers of poly(d,l-lactide) obtained by solution blow spinning: Process and solution variables. J. Appl. Polym. Sci. 2011, 122, 3396–3405. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Moraes, E.A.; Marconcini, J.M.; Mattoso, L.H.; Glenn, G.M.; Medeiros, E.S. Properties of poly(lactic acid) and poly(ethylene oxide) solvent polymer mixtures and nanofibers made by solution blow spinning. J. Appl. Polym. Sci. 2013, 129, 3672–3681. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Bilbao-Sainz, C.; Du, W.-X.; Chiou, B.-S.; Williams, T.G.; Wood, D.F.; Imam, S.H.; Orts, W.J.; Lopez-Rubio, A.; Lagaron, J.M. Antimicrobial poly (lactic acid)-based nanofibres developed by solution blow spinning. J. Nanosci. Nanotechnol. 2015, 15, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Avena-Bustillos, R.J.; Bilbao-Sainz, C.; Woods, R.; Chiou, B.S.; Wood, D.; Williams, T.; Yokoyama, W.; Glenn, G.M.; McHugh, T.H.; et al. Solution blow spinning of food-grade gelatin nanofibers. J. Food Sci. 2017, 82, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhong, Q. Blending lecithin and gelatin improves the formation of thymol nanodispersions. J. Agric. Food Chem. 2014, 62, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical properties and antimicrobial efficacy of carvacrol nanoemulsions formed by spontaneous emulsification. J. Agric. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Electrospinning chitosan/poly(ethylene oxide) solutions with essential oils: Correlating solution rheology to nanofiber formation. Carbohydr. Polym. 2016, 139, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.; Kleinmeyer, J.; Harris, D.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Kriegel, C.; Kit, K.M.; McClements, D.J.; Weiss, J. Nanofibers as carrier systems for antimicrobial microemulsions. part I: Fabrication and characterization. Langmuir 2009, 25, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Xu, F.; Gao, Y.; Li, H.; Chen, D. Macrocellular polymer foams from water in oil high internal phase emulsion stabilized solely by polymer Janus nanoparticles: Preparation and their application as support for Pd catalyst. RSC Adv. 2015, 5, 40227–40235. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Parize, D.D.D.S.; Foschini, M.M.; Oliveira, J.E.D.; Klamczynski, A.P.; Glenn, G.M.; Marconcini, J.M.; Mattoso, L.H.C. Solution blow spinning: Parameters optimization and effects on the properties of nanofibers from poly(lactic acid)/dimethyl carbonate solutions. J. Mater. Sci. 2016, 51, 4627–4638. [Google Scholar] [CrossRef]
- Parize, D.D.D.S.; Oliveira, J.E.D.; Foschini, M.M.; Marconcini, J.M.; Mattoso, L.H.C. Poly(lactic acid) fibers obtained by solution blow spinning: Effect of a greener solvent on the fiber diameter. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Structural and morphological characterization of micro and nanofibers produced by electrospinning and solution blow spinning: A comparative study. Adv. Mater. Sci. Eng. 2013, 2013, 409572. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of film from splendid squid (Loligo formosana) skin gelatin with various extraction temperatures. Int. J. Biol. Macromol. 2012, 51, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Ye, R.; Wang, W.; Li, X. Transglutaminase-induced crosslinking of gelatin-calcium carbonate composite films. Food Chem. 2015, 166, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, X.; Zhang, R.; Lan, W.; Qin, W. Fabrication of Electrospun polylactic acid/cinnamaldehyde/β-cyclodextrin fibers as an antimicrobial wound dressing. Polymers 2017, 9, 464. [Google Scholar] [CrossRef]
- Al-Bayati, F.A.; Mohammed, M.J. Isolation, identification, and purification of cinnamaldehyde fromCinnamomum zeylanicumbark oil. An antibacterial study. Pharm. Biol. 2009, 47, 61–66. [Google Scholar] [CrossRef]
- Manu, D.; Mendonca, A.F.; Daraba, A.; Dickson, J.S.; Sebranek, J.; Shaw, A.; Wang, F.; White, S. Antimicrobial efficacy of cinnamaldehyde against Escherichia coli O157:H7 and salmonella enterica in carrot juice and mixed berry juice held at 4 °C and 12 °C. Foodborne Pathog. Dis. 2017, 14, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Moura, M.R.D.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.D.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Kurek, M.; Guinault, A.; Voilley, A.; Galic, K.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Dogan, S.Y.; Tekinay, T.; Uyar, T. Release and antibacterial activity of allyl isothiocyanate/β-cyclodextrin complex encapsulated in electrospun nanofibers. Colloids Surf. B Biointerfaces 2014, 120, 125–131. [Google Scholar] [CrossRef] [PubMed]








| Samples | Cinnamaldehyde Retention after Fiber Preparation (mg C/mg F) | Cinnamaldehyde Retention after Film Preparation (mg C/mg F) |
|---|---|---|
| Blank | - | - |
| FFE-5 | 0.013 | 0.002 |
| FFE-10 | 0.004 | 0.044 |
| FFE-15 | 0.012 | 0.021 |
| FFE-20 | 0.058 | 0.074 |
| FFE-25 | 0.080 | 0.116 |
| FFE-30 | 0.084 | 0.171 |
| Ingredient (g) | Blank | FFE-5 | FFE-10 | FFE-15 | FFE-20 | FFE-25 | FFE-30 |
|---|---|---|---|---|---|---|---|
| FSG | 19.8 | 19.8 | 19.8 | 19.8 | 19.8 | 19.8 | 19.8 |
| 20% acetic acid | 100.2 | 96.24 | 95.25 | 94.26 | 93.27 | 92.28 | 91.29 |
| Cinnamaldehyde | - | 0.99 | 1.98 | 2.97 | 3.96 | 4.95 | 5.94 |
| Lecithin | - | 2.97 | 2.97 | 2.97 | 2.97 | 2.97 | 2.97 |
| Total | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Türker Saricaoglu, F.; Avena-Bustillos, R.J.; Bridges, D.F.; Takeoka, G.R.; Wu, V.C.H.; Chiou, B.-S.; Wood, D.F.; McHugh, T.H.; Zhong, F. Preparation of Fish Skin Gelatin-Based Nanofibers Incorporating Cinnamaldehyde by Solution Blow Spinning. Int. J. Mol. Sci. 2018, 19, 618. https://doi.org/10.3390/ijms19020618
Liu F, Türker Saricaoglu F, Avena-Bustillos RJ, Bridges DF, Takeoka GR, Wu VCH, Chiou B-S, Wood DF, McHugh TH, Zhong F. Preparation of Fish Skin Gelatin-Based Nanofibers Incorporating Cinnamaldehyde by Solution Blow Spinning. International Journal of Molecular Sciences. 2018; 19(2):618. https://doi.org/10.3390/ijms19020618
Chicago/Turabian StyleLiu, Fei, Furkan Türker Saricaoglu, Roberto J. Avena-Bustillos, David F. Bridges, Gary R. Takeoka, Vivian C. H. Wu, Bor-Sen Chiou, Delilah F. Wood, Tara H. McHugh, and Fang Zhong. 2018. "Preparation of Fish Skin Gelatin-Based Nanofibers Incorporating Cinnamaldehyde by Solution Blow Spinning" International Journal of Molecular Sciences 19, no. 2: 618. https://doi.org/10.3390/ijms19020618
APA StyleLiu, F., Türker Saricaoglu, F., Avena-Bustillos, R. J., Bridges, D. F., Takeoka, G. R., Wu, V. C. H., Chiou, B.-S., Wood, D. F., McHugh, T. H., & Zhong, F. (2018). Preparation of Fish Skin Gelatin-Based Nanofibers Incorporating Cinnamaldehyde by Solution Blow Spinning. International Journal of Molecular Sciences, 19(2), 618. https://doi.org/10.3390/ijms19020618