Disruption of Central Antioxidant Property of Nuclear Factor Erythroid 2-Related Factor 2 Worsens Circulatory Homeostasis with Baroreflex Dysfunction in Heart Failure
Abstract
:1. Introduction
2. Results
2.1. Assessment of Myocardial Infarction and Heart Failure
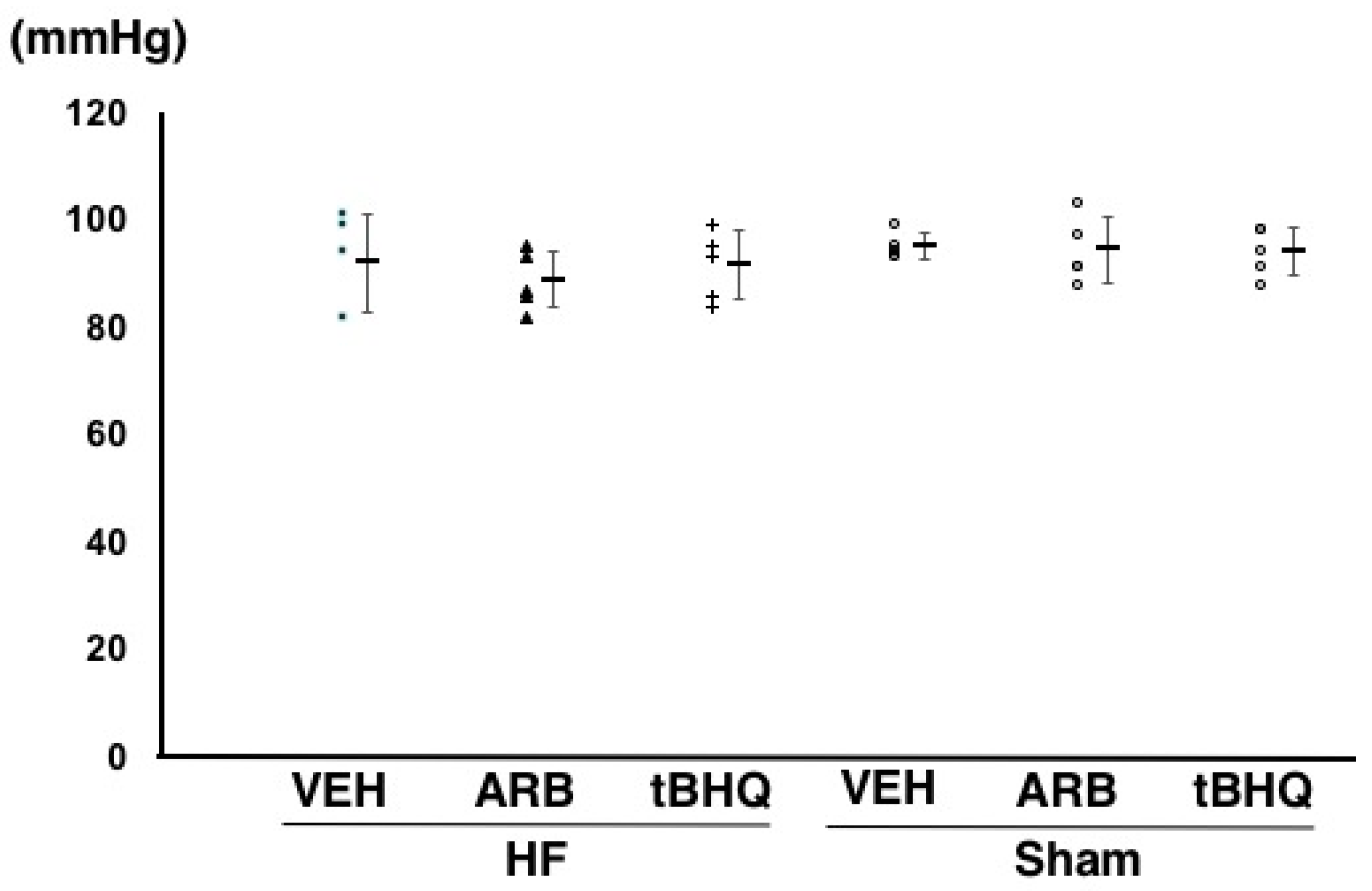
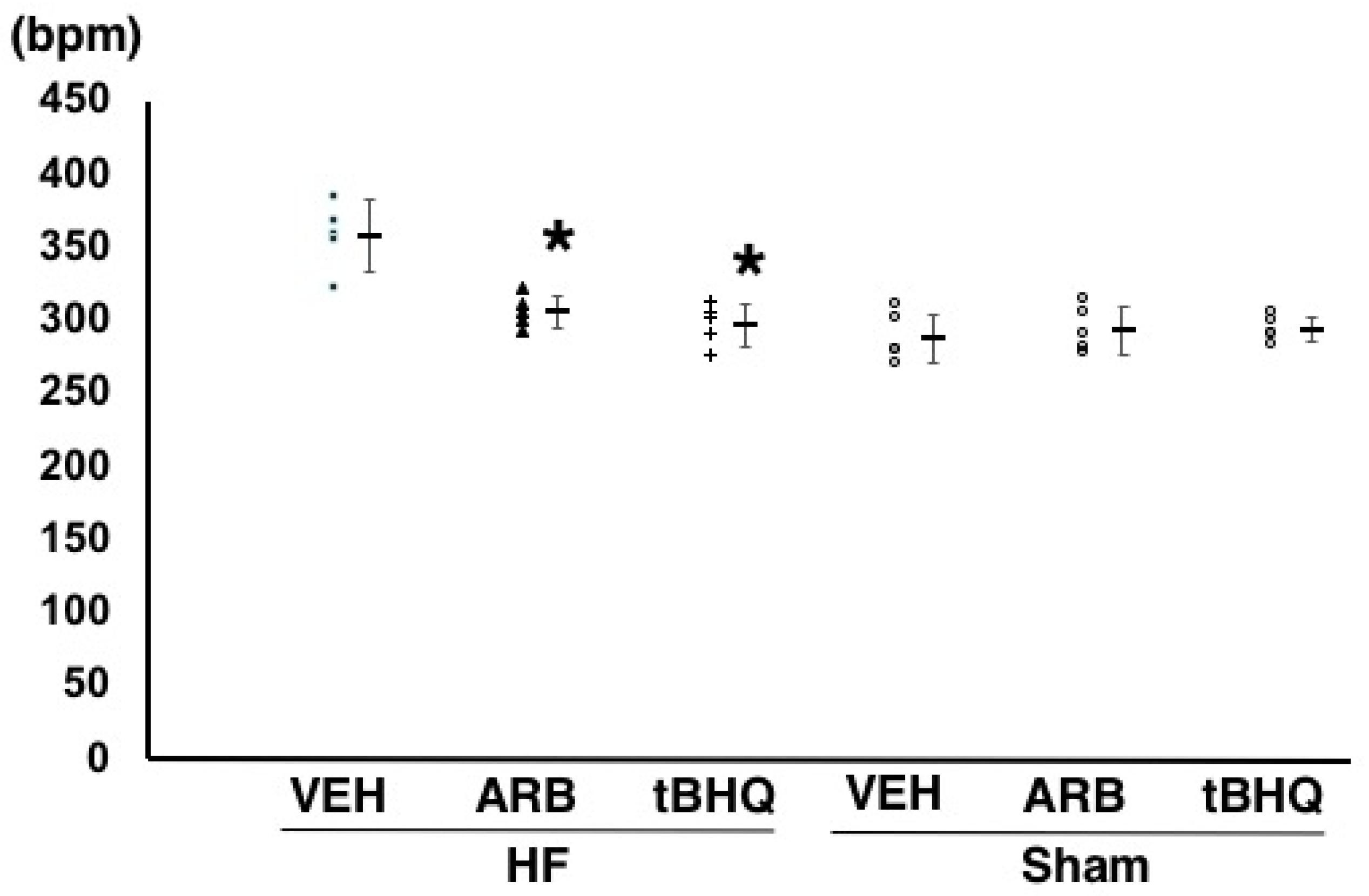
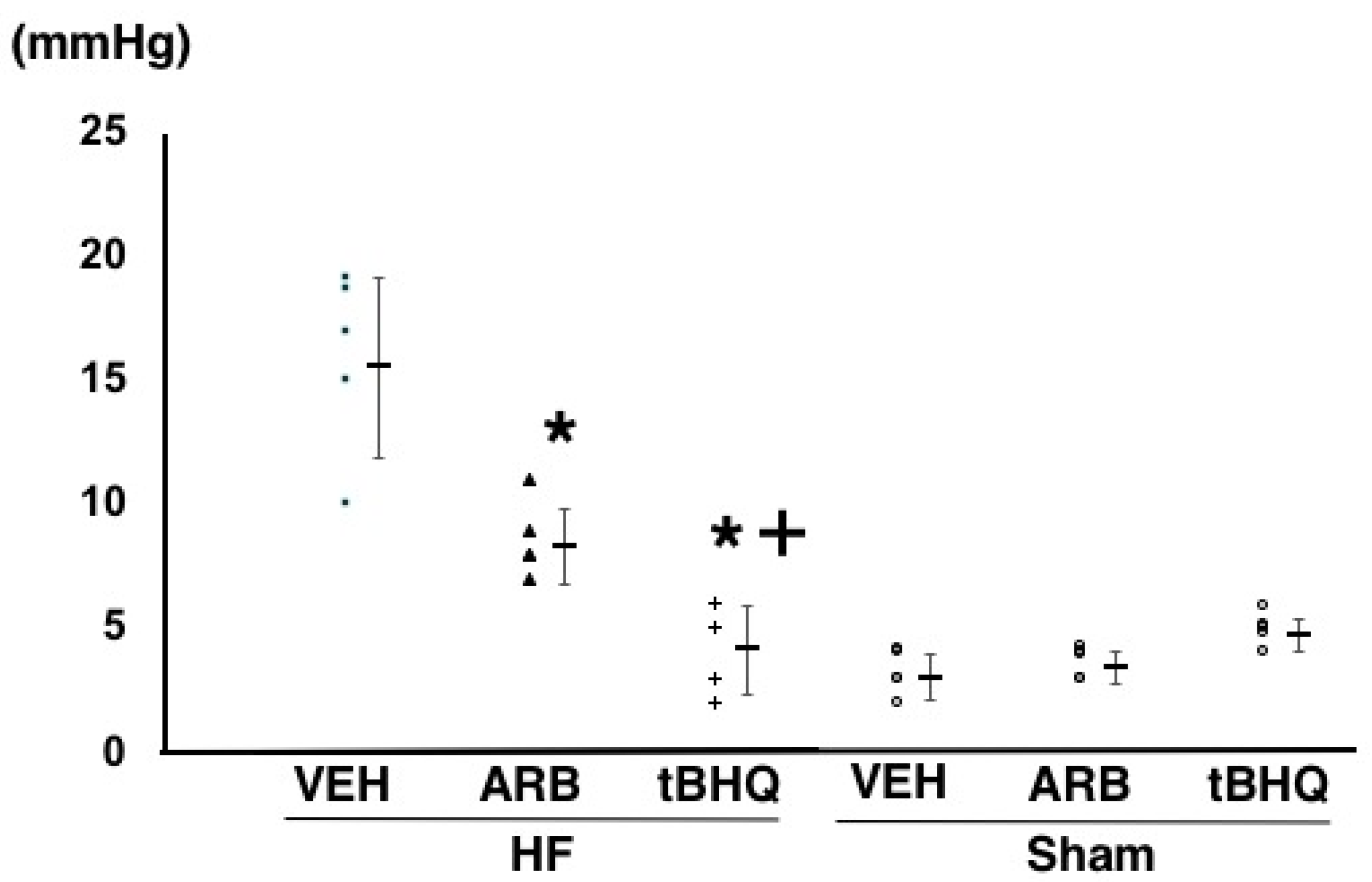
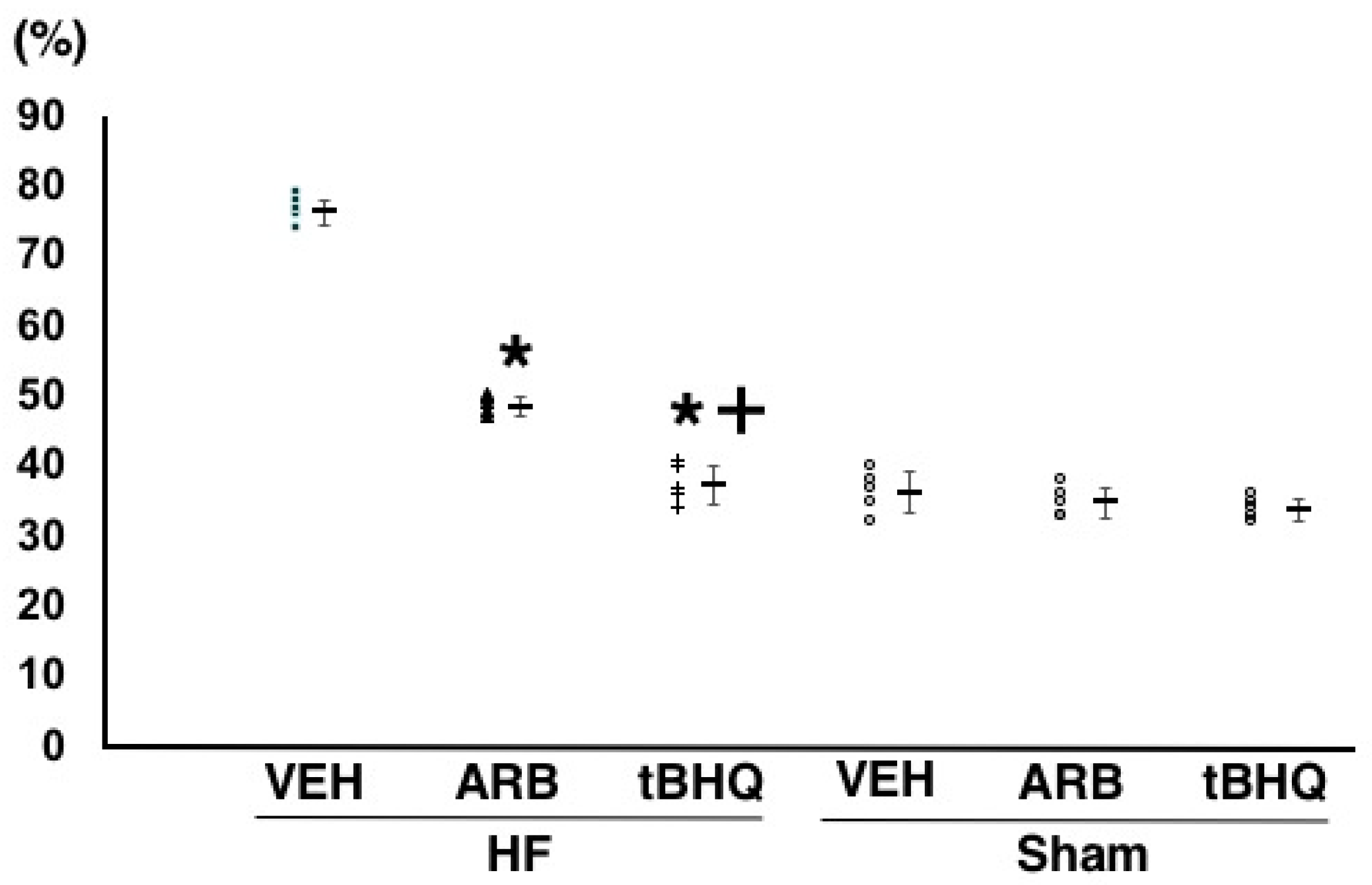
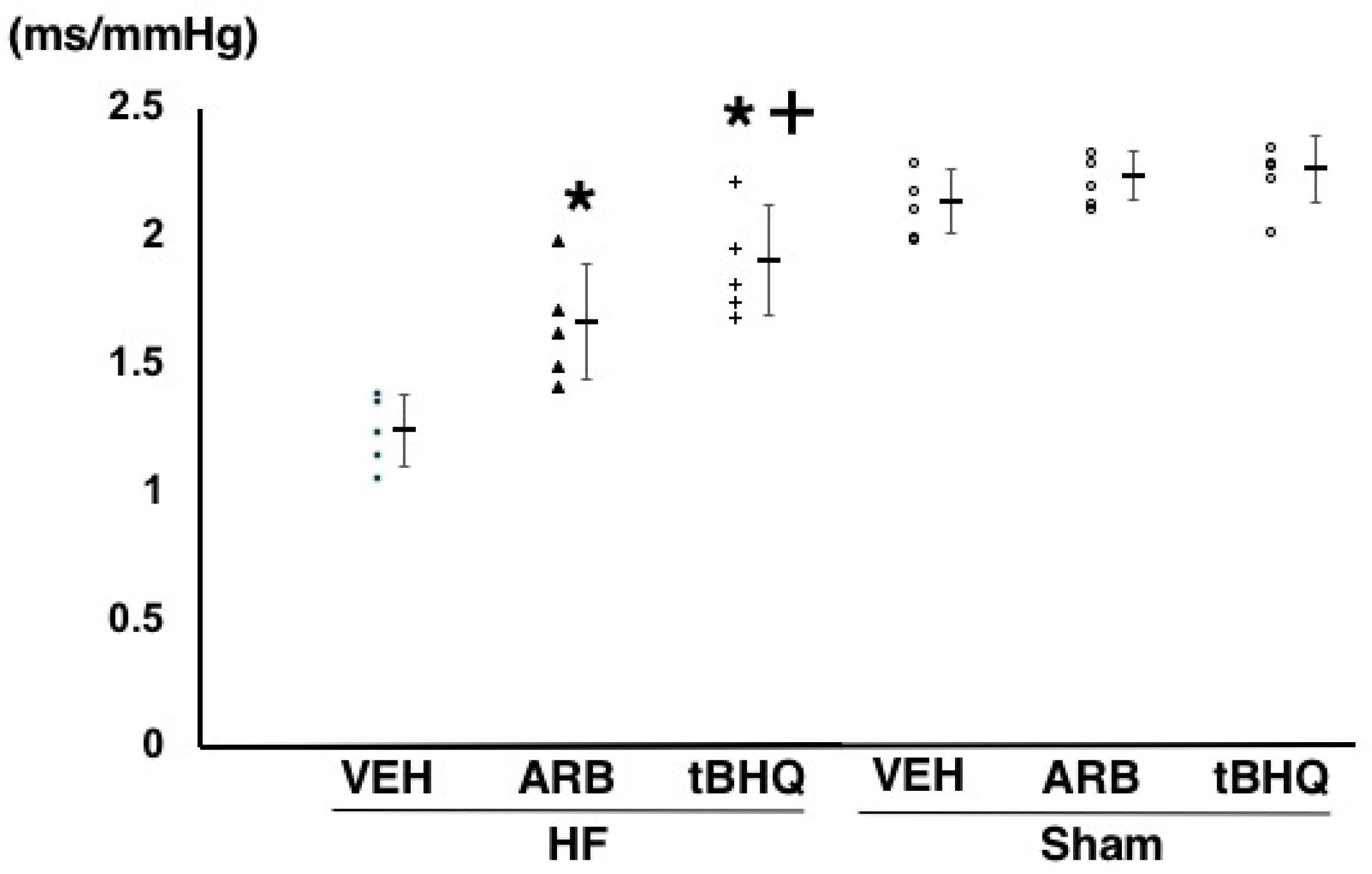
2.2. Hemodynamics, Autonomic Function, and Baroreflex Sensitivity
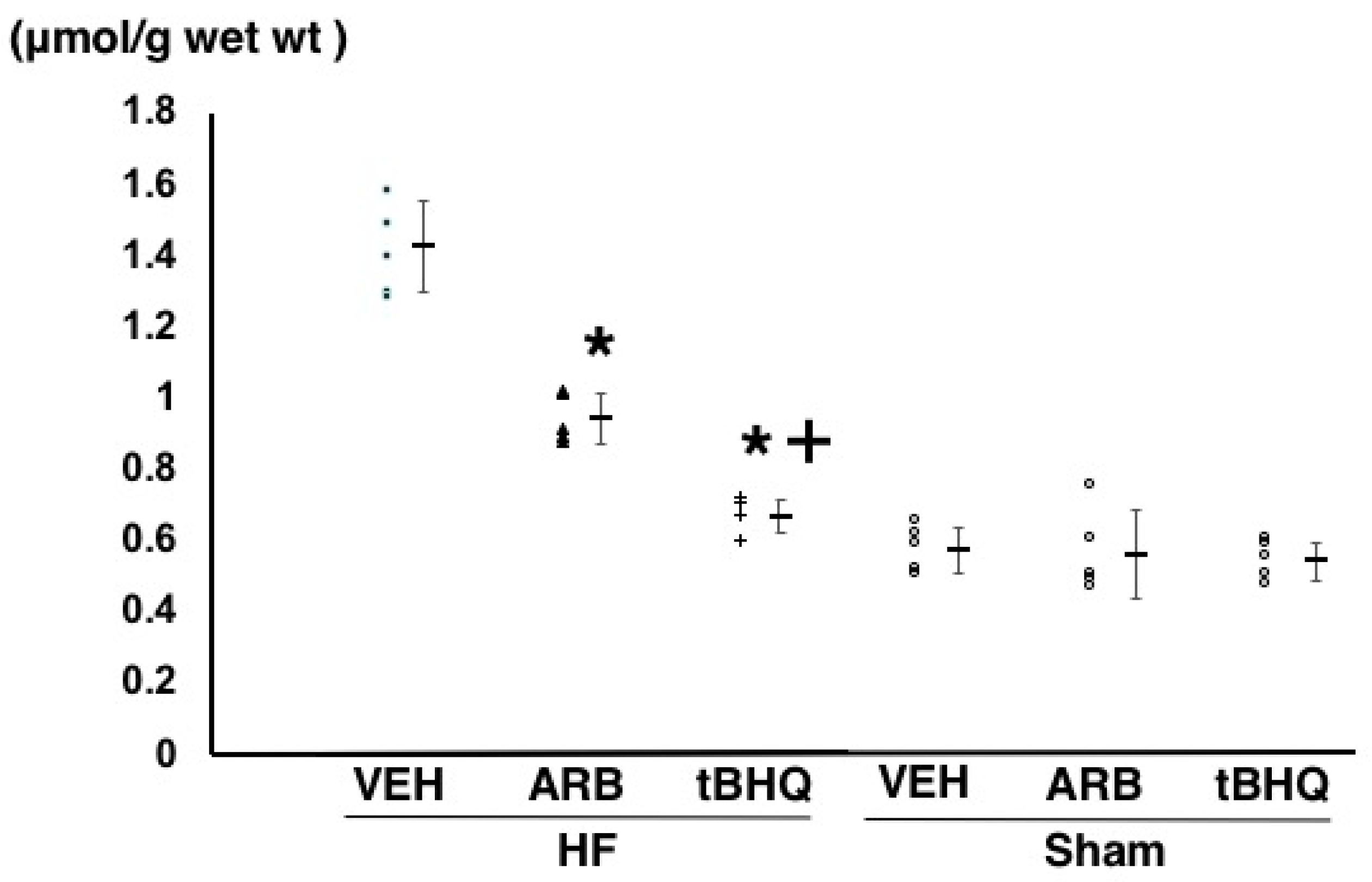
2.3. Oxidative Stress in the RVLM
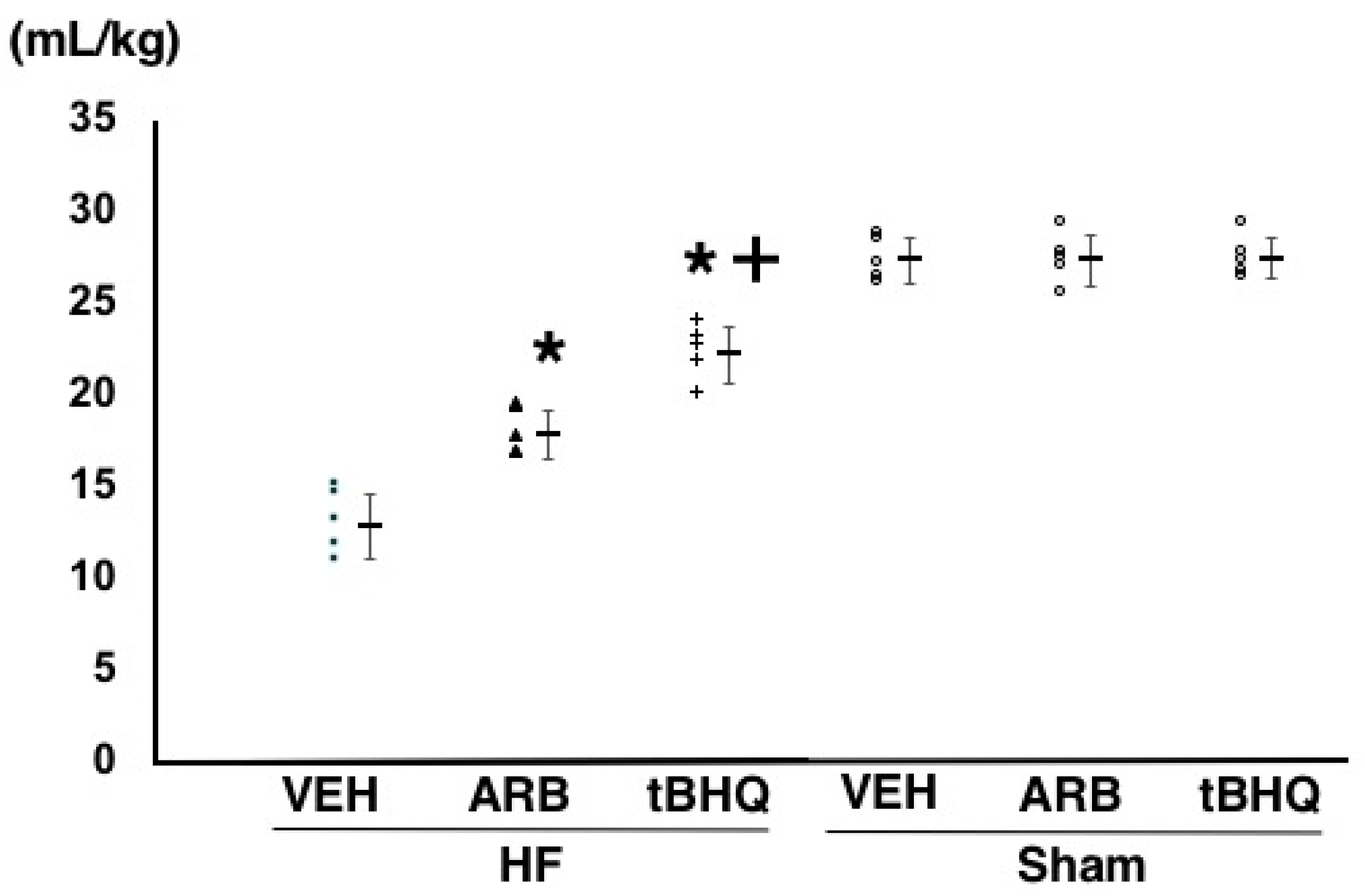
2.4. Circulatory Homeostasis Assessed by Volume Tolerance
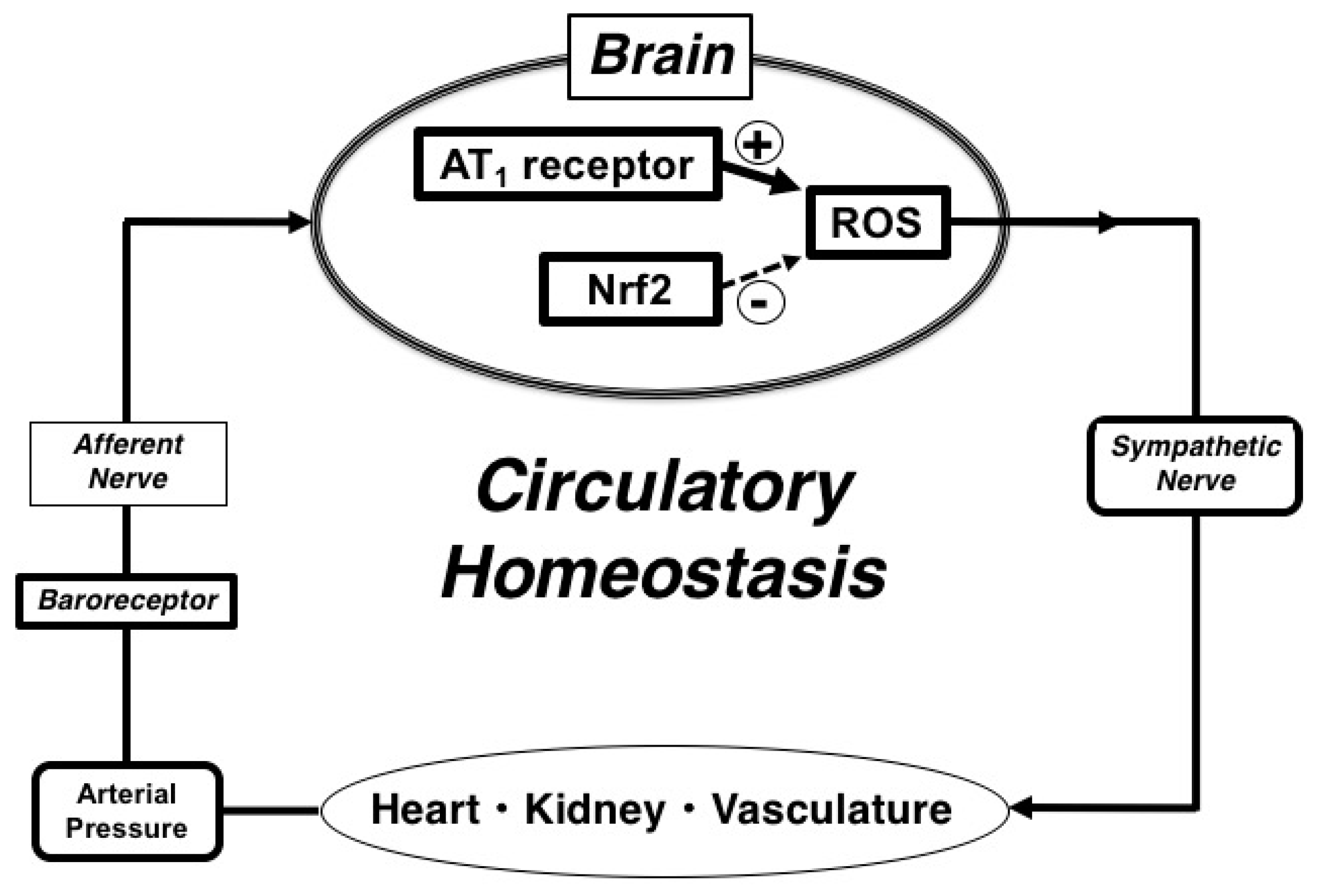
3. Discussion
4. Materials and Methods
4.1. Animals and General Procedures
4.2. Assessment of Hemodynamics, Autonomic Function, and Baroreflex Function
4.3. Measurement of Thiobarbituric Acid-Reactive Substances in the RVLM
4.4. Assessment of Volume Tolerance
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grassi, G. Sympathetic neural activity in human hypertension and related diseases. Am. J. Hypertens. 2010, 23, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T. Heart failure as an autonomic nervous system dysfunction. J. Cardiol. 2012, 59, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Floras, J.S. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. J. Am. Coll. Cardiol. 2009, 54, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Dampney, R.A.L. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Kimura, Y.; Ito, K.; Shimokawa, H.; Takeshita, A. Increased reactive oxygen species in rostral ventrolateral medulla contributes to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 2004, 109, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Konno, S.; Ogawa, K.; Sunagawa, K. Angiotensin II type 1 receptor activated caspase-3 through ras/mitogen-activated protein kinase/extracellular signal regulated kinase in the rostral ventrolateral medulla is involved in sympathoexcitation in stroke-prone spontaneously hypertensive rats. Hypertension. 2010, 55, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T. Regulation of sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 academic conference award from the Japanese society of hypertension. Hypertens. Res. 2013, 36, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, K.; Hosokawa, K.; Kishi, T.; Ide, T.; Sunagawa, K. Striking volume intolerance is induced by mimicking arterial baroreflex failure in normal left ventricular function. J. Card. Fail. 2014, 20, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Kakino, T.; Sakamoto, K.; Tobushi, T.; Tanaka, A.; Saku, K.; Hosokawa, K.; Onitsuka, K.; Murayama, Y.; Tsutsumi, T.; et al. Changes in vascular properties, not ventricular properties, predominantly contribute to baroreflex regulation of arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H49–H58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, T. Heart failure as a disruption of dynamic circulatory homeostasis mediated by brain. Int. Heart J. 2016, 57, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Habas, A.; Hahn, J.; Wang, X.; Margeta, M. Neuronal activity regulates astrocytic Nrf2 signaling. Proc. Nalt. Acad. Sci. USA 2013, 110, 18291–18296. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 4, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [PubMed]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Nalt. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.R.; Johnson, D.A.; Sirkis, D.W.; Messing, A.; Johnson, J.A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008, 28, 13574–13581. [Google Scholar] [CrossRef] [PubMed]
- Isegawa, K.; Hirooka, Y.; Katsuki, M.; Kishi, T.; Sunagawa, K. Targeting deletion of astrocyte-specific angiotensin II type 1 receptors improves prognosis after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1448–H1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, K.; Kishi, T.; Hirooka, Y.; Sunagawa, K. Circulating angiotensin II deteriorates left ventricular function with sympathoexcitation via brain angiotensin II receptor. Physiol. Rep. 2015, 3, e12514. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Sunagawa, K. Telmisartan reduces mortality and left ventricular hypertrophy with sympathoinhibition in rats with hypertension and heart failure. Am. J. Hypertens. 2014, 27, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J.R. Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration? Antiosidant 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, A.; Sakamoto, K.; Saku, K.; Akashi, T.; Murayama, Y.; Kishi, T.; Ide, T.; Sunagawa, K. Optimal titration is important to maximize the beneficial effects of vagal nerve stimulation in chronic heart failure. J. Card. Fail. 2016, 22, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, T.; Hirooka, Y.; Kishi, T.; Mukai, Y.; Inoue, S.; Takase, S.; Takemoto, M.; Chishaki, A.; Sunagawa, K. Blockade of brain angiotensin II type 1 receptor inhibits development of atrial fibrillation in hypertensive rats. Am. J. Hypertens. 2015, 28, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Li, P.; Murphy, T.H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Hosokawa, K.; Saku, K.; Sakamoto, T.; Tobushi, T.; Oga, Y.; Kishi, T.; Ide, T.; Sunagawa, K. Baroreflex failure predisposes to pulmonary edema in conscious rats with normal left ventricular function. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H199–H205. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishi, T. Disruption of Central Antioxidant Property of Nuclear Factor Erythroid 2-Related Factor 2 Worsens Circulatory Homeostasis with Baroreflex Dysfunction in Heart Failure. Int. J. Mol. Sci. 2018, 19, 646. https://doi.org/10.3390/ijms19030646
Kishi T. Disruption of Central Antioxidant Property of Nuclear Factor Erythroid 2-Related Factor 2 Worsens Circulatory Homeostasis with Baroreflex Dysfunction in Heart Failure. International Journal of Molecular Sciences. 2018; 19(3):646. https://doi.org/10.3390/ijms19030646
Chicago/Turabian StyleKishi, Takuya. 2018. "Disruption of Central Antioxidant Property of Nuclear Factor Erythroid 2-Related Factor 2 Worsens Circulatory Homeostasis with Baroreflex Dysfunction in Heart Failure" International Journal of Molecular Sciences 19, no. 3: 646. https://doi.org/10.3390/ijms19030646



