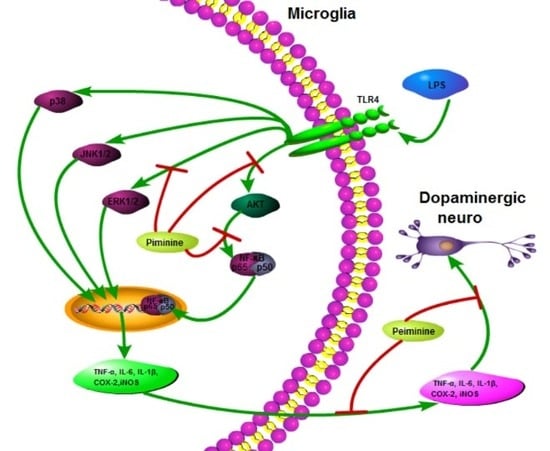
Peiminine Protects Dopaminergic Neurons from Inflammation-Induced Cell Death by Inhibiting the ERK1/2 and NF-κB Signalling Pathways
Abstract
:1. Introduction
2. Results
2.1. Peiminine Improves LPS-Induced Behavioural Dysfunction
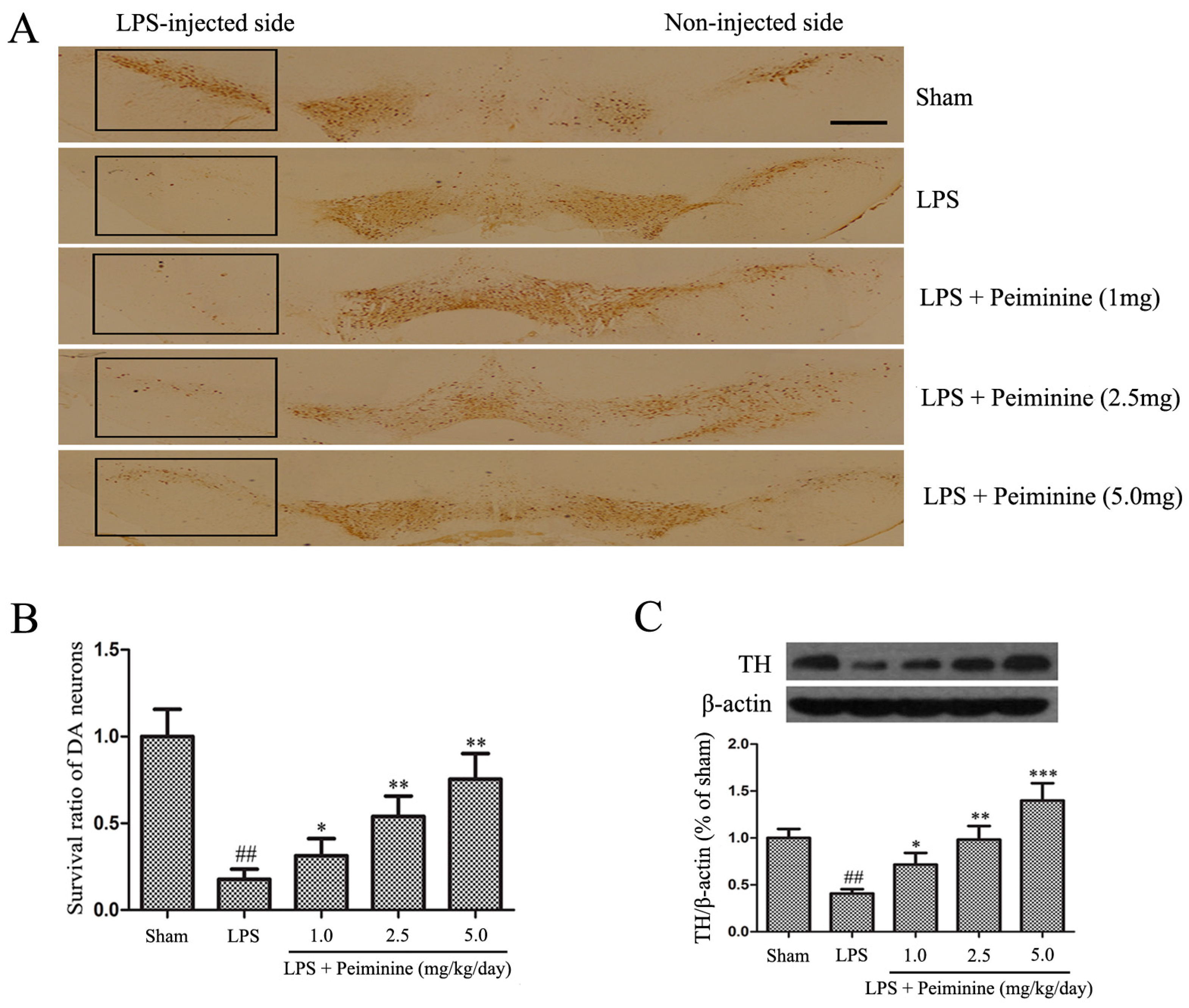
2.2. Peiminine Inhibits the LPS-Induced Loss of Dopaminergic Neurons in the SNpc
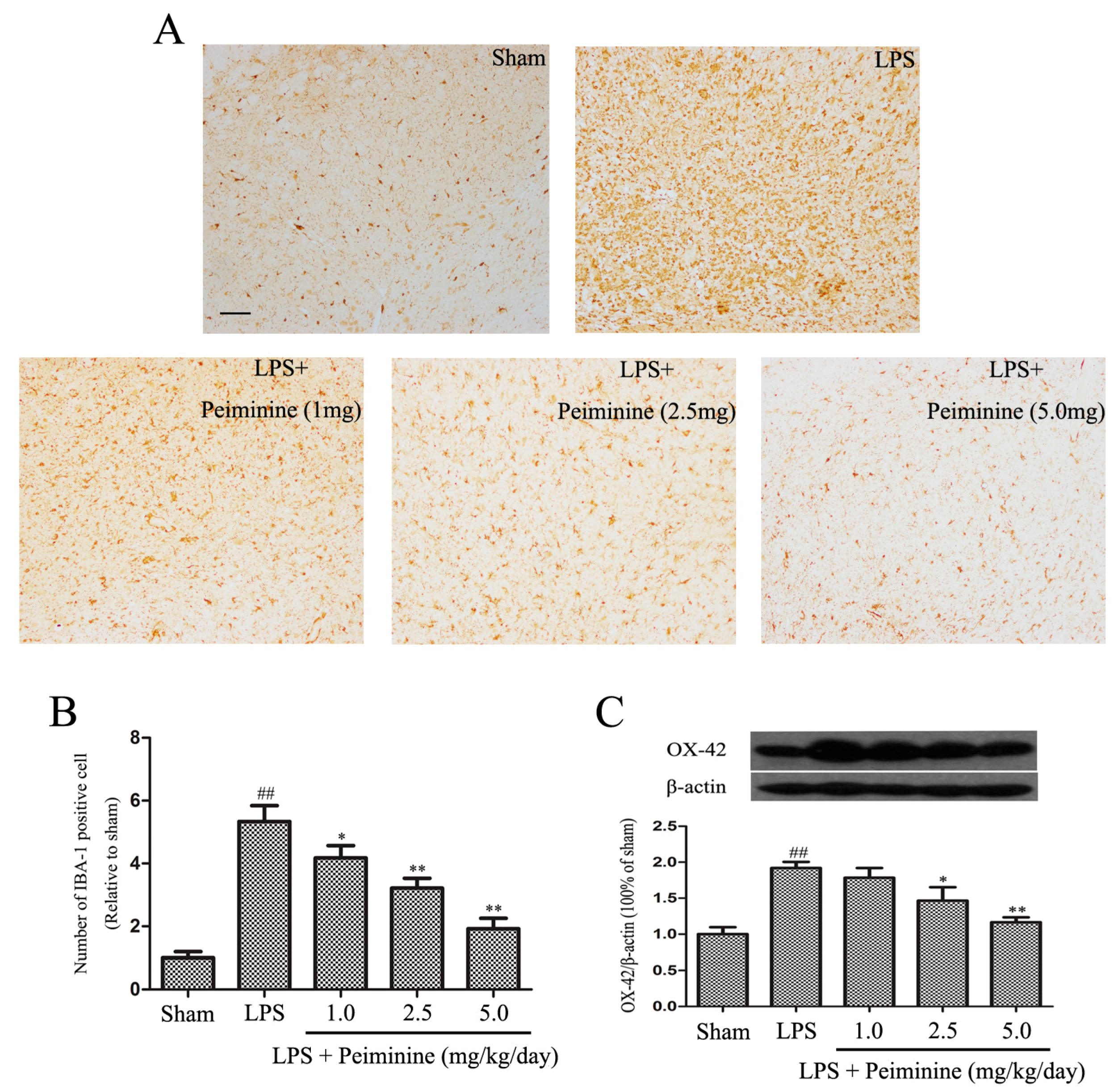
2.3. Peiminine Suppresses LPS-Induced Microglia Activation
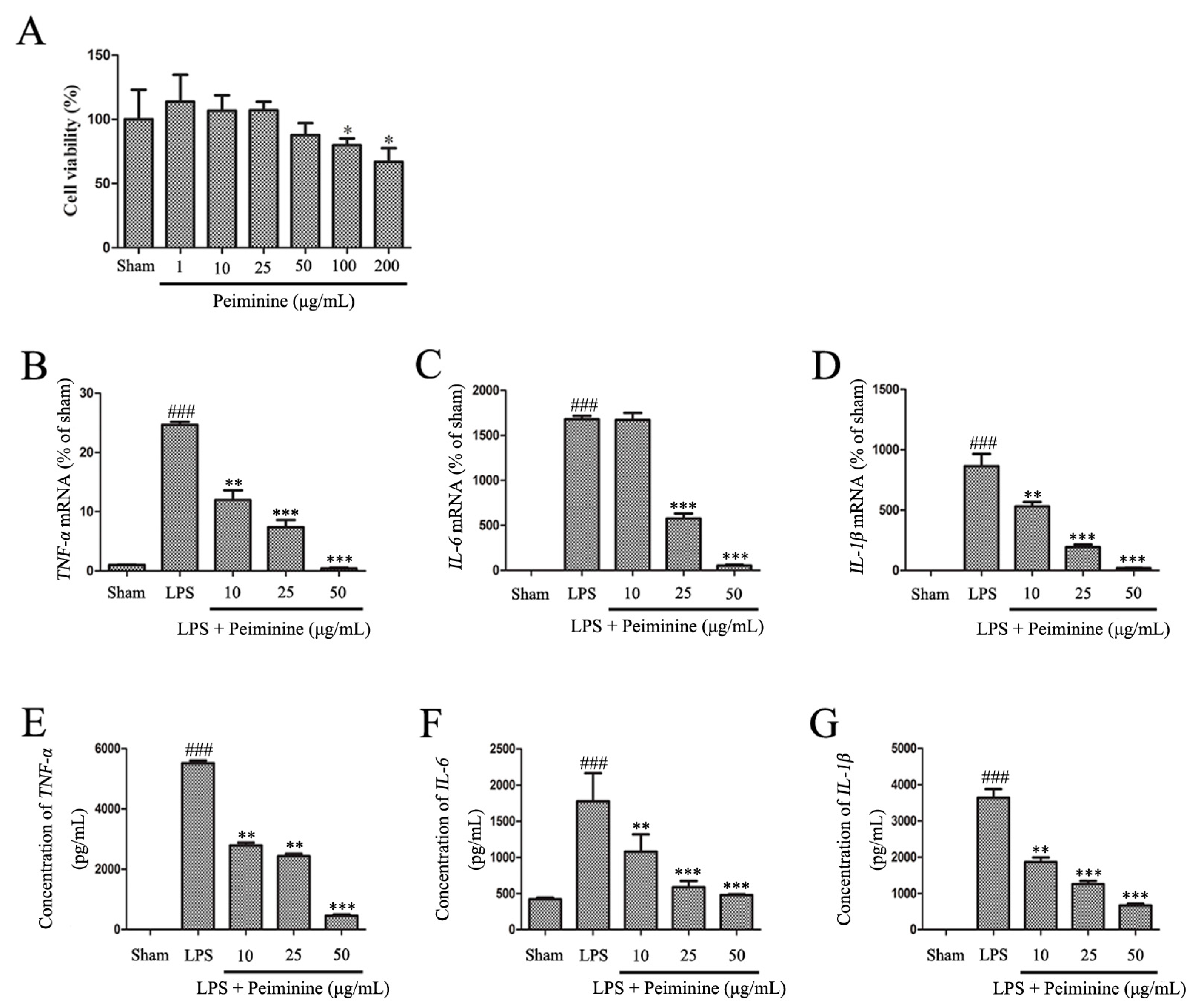
2.4. Peiminine Inhibits the LPS-Induced Inflammatory Response in BV-2 Cells
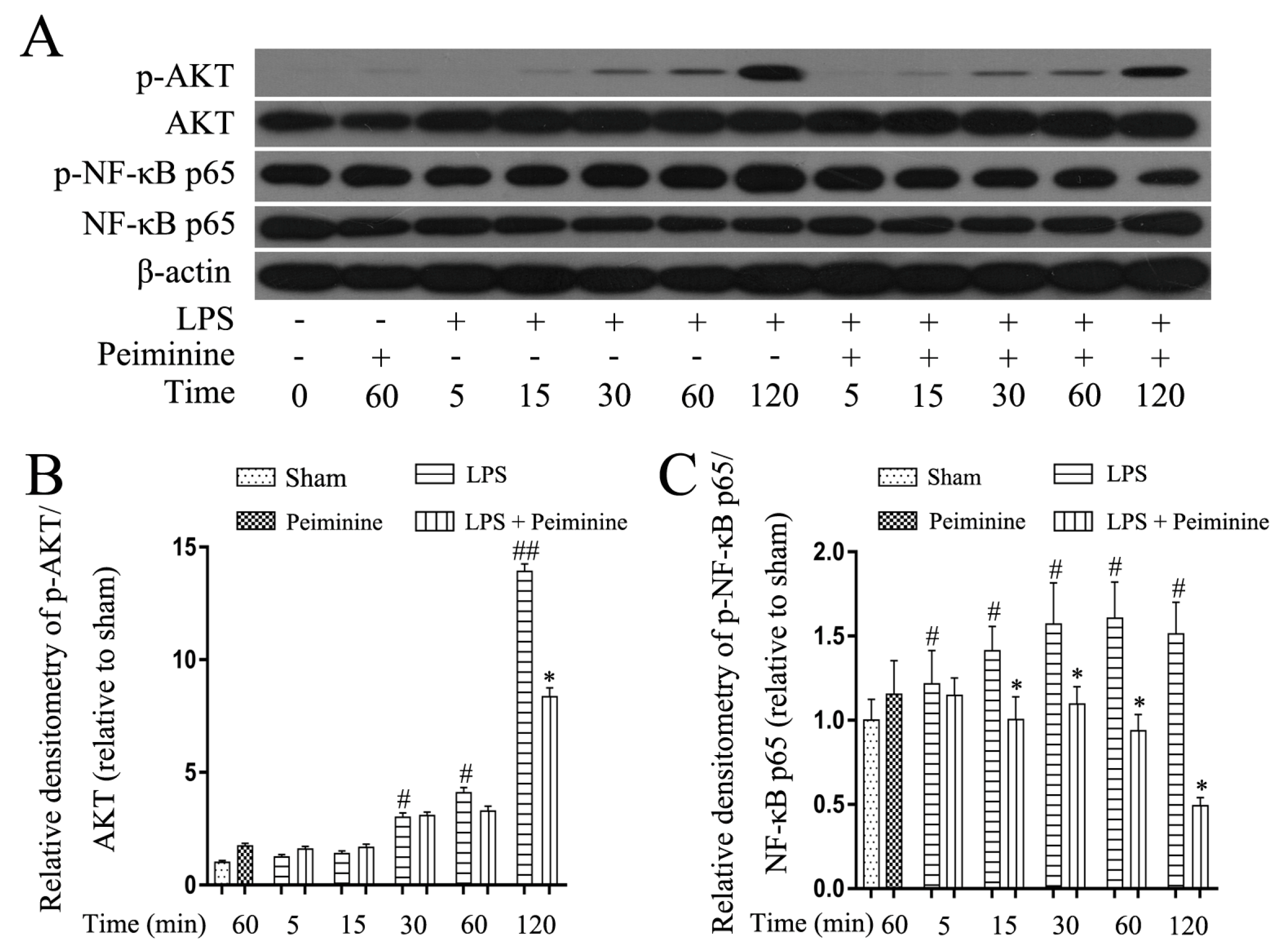
2.5. Peiminine Suppresses LPS-Induced NF-κB p65 Phosphorylation in BV-2 Cells
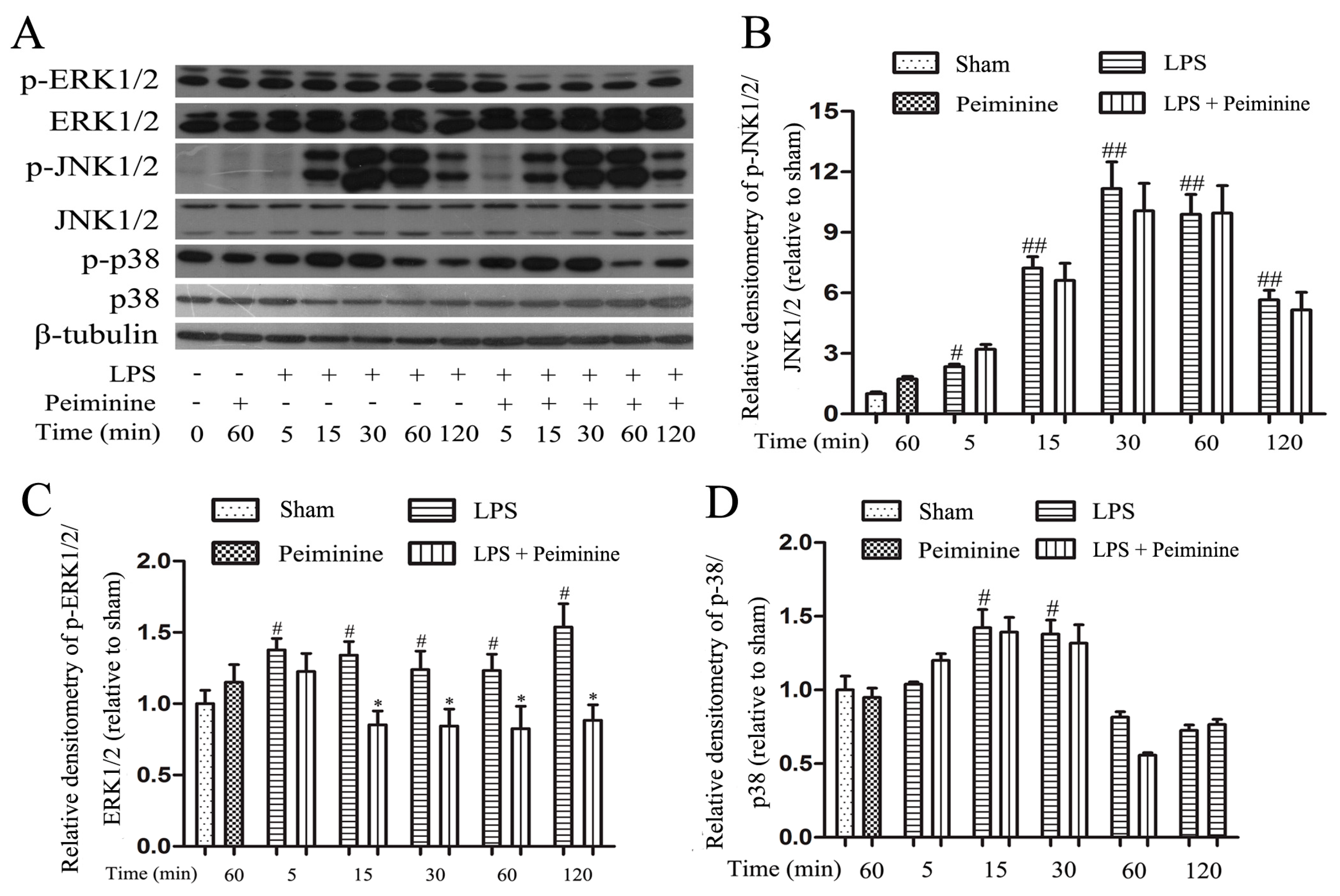
2.6. Peiminine Suppresses LPS-Induced ERK1/2 Phosphorylation in BV-2 Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Rotational Behaviour Assay
4.3. Cells and Treatments
4.4. Immunohistological Analysis
4.5. Cell Viability Assay
4.6. Quantitative Real-Time PCR
4.7. ELISA
4.8. Western Blot Analysis
4.9. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| SNpc | Substantia nigra pars compacta |
| TH | Tyrosine hydroxylase |
| IBA-1 | Ionized calcium-binding adaptor molecule-1 |
References
- Kang, J.; Chung, K.C. The F-box protein FBXO7 positively regulates bone morphogenetic protein-mediated signaling through Lys-63-specific ubiquitination of neurotrophin receptor-interacting MAGE (NRAGE). Cell. Mol. Life Sci. 2015, 72, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Tatton, W.G. Etiology and pathogenesis of Parkinson’s disease. Ann. Rev. Neurosci. 1999, 22, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Ann. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Pascual, C.; Perez-Gonzalez, R.; Orive, G.; Carro, E. Intranasal PRGF-Endoret enhances neuronal survival and attenuates NF- κB-dependent inflammation process in a mouse model of Parkinson’s disease. J. Control. Release 2015, 203, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Zipp, F.; Aktas, O. The brain as a target of inflammation: Common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006, 29, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [PubMed]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; Lopez-Cuenca, I.; Rojas, P.; Trivino, A.; Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef]
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Li, P.; Li, S.L.; Chan, S.W. Chromatographic analysis of Fritillaria isosteroidal alkaloids, the active ingredients of Beimu, the antitussive traditional Chinese medicinal herb. J. Chromatogr. A 2001, 935, 321–338. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, Y.X.; Lin, J.; Xu, Y.; Yan, F.; Tang, L.; Chen, F. Identification of bulb from Fritillaria cirrhosa by PCR with specific primers. Planta Med. 2003, 69, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Q.; Gao, L.-M.; Yang, Y.-P. Genetic diversity and structure of a traditional Chinese medicinal plant species, Fritillaria cirrhosa (Liliaceae) in southwest China and implications for its conservation. Biochem. Syst. Ecol. 2010, 38, 236–242. [Google Scholar] [CrossRef]
- Guo, H.; Ji, F.; Liu, B.; Chen, X.; He, J.; Gong, J. Peiminine ameliorates bleomycin-induced acute lung injury in rats. Mol. Med. Rep. 2013, 7, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, X.; Song, J.; Wang, L.; Chen, D.; Yang, Y.; Bai, X.; Wang, J.; Shi, Y.; Chen, S. Therapeutic effects of traditional Chinese medicine Niubeixiaohe in mouse tuberculosis models. J. Ethnopharmacol. 2017, 195, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, J.; Du, Q.; Li, H.; Wang, S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J. Ethnopharmacol. 2016, 193, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Z.; Zhang, L.; Atanasov, A.G.; Wang, S. Characterization of the Isosteroidal Alkaloid Chuanbeinone from Bulbus of Fritillaria pallidiflora as Novel Antitumor Agent In Vitro and In Vivo. Planta Med. 2016, 82, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, E.Y.; Kim, J.H.; Min, J.H.; Jeong, D.W.; Jun, J.Y.; Cho, C.Y.; Sohn, Y.; Jung, H.S. Antiallergic effects of peiminine through the regulation of inflammatory mediators in HMC-1 cells. Immunopharmacol. Immunotoxicol. 2015, 37, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Mo, C.; Xiao, H.; Jiang, Y.; Ye, B.; Wang, S. Imperialine and Verticinone from Bulbs of Fritillaria wabuensis Inhibit Pro-inflammatory Mediators in LPS-stimulated RAW 264.7 Macrophages. Planta Med. 2015, 81, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.M.; Liu, B.R.; Zeng, Y.L.; Li, S.N.; Huang, B.X.; Lv, Q.K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Levitt, M.; Udenfriend, S. Tyrosine Hydroxylase. The Initial Step in Norepinephrine Biosynthesis. J. Biol. Chem. 1964, 239, 2910–2917. [Google Scholar] [PubMed]
- Hirsch, E.C.; Hunot, S.; Hartmann, A. Neuroinflammatory processes in Parkinson’s disease. Parkinsonism Relat. Disord. 2005, 11 (Suppl. S1), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Liu, Y.; Liu, S.; Cao, S.; Wang, F.; Wang, Z.; Xi, S. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol. Neurobiol. 2016, 53, 4449–4460. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, B.W.; Ren, W.Z.; Liu, J.X.; Li, S.N.; Fu, S.P.; Zeng, Y.L.; Xu, S.Y.; Yan, X.; Gao, Y.J.; et al. GLP-2 Attenuates LPS-Induced Inflammation in BV-2 Cells by Inhibiting ERK1/2, JNK1/2 and NF- κB Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, D.F.; Zhang, X.Y.; Liu, D.; Xu, S.Y.; Chen, G.X.; Huang, B.X.; Ren, W.Z.; Wang, W.; Fu, S.P.; et al. Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. History of innate immunity in neurodegenerative disorders. Front. Pharmacol. 2011, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Sprenkle, N.T.; Sims, S.G.; Sanchez, C.L.; Meares, G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, R.; Zhu, S.; Zhang, R.; Ma, S. Rhynchophylline attenuates LPS-induced pro-inflammatory responses through down-regulation of MAPK/NF-κB signaling pathways in primary microglia. Phytother. Res. 2012, 26, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.; Candelario-Jalil, E.; Bhatia, H.S.; Lieb, K.; Hull, M.; Fiebich, B.L. Regulation of prostaglandin E2 synthase expression in activated primary rat microglia: Evidence for uncoupled regulation of mPGES-1 and COX-2. Glia 2008, 56, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Fu, S. Galangin Reduces the Loss of Dopaminergic Neurons in an LPS-Evoked Model of Parkinson’s Disease in Rats. Int. J. Mol. Sci. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Ju, C.; Yang, D.; Chen, G.; Xu, S.; Zeng, Y.; Yan, X.; Wang, W.; Liu, D.; et al. Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide (LPS)-Induced Parkinson’s Disease Models. Int. J. Mol. Sci. 2017, 18, 2043. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 2003, 278, 34783–34793. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Jorge-Finnigan, A.; Kleppe, R.; Jung-Kc, K.; Ying, M.; Marie, M.; Rios-Mondragon, I.; Salvatore, M.F.; Saraste, J.; Martinez, A. Phosphorylation at serine 31 targets tyrosine hydroxylase to vesicles for transport along microtubules. J. Biol. Chem. 2017, 292, 14092–14107. [Google Scholar] [CrossRef] [PubMed]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Ye, X.; Bao, X.; Zhao, B.; Wang, X.; Zhang, D. Inhibition of Src tyrosine kinase activity by squamosamide derivative FLZ attenuates neuroinflammation in both in vivo and in vitro Parkinson’s disease models. Neuropharmacology 2013, 75, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Liu, X.Y.; Niu, D.B.; Li, F.Q.; He, Q.H.; Wang, X.M. Triptolide protects dopaminergic neurons from inflammation-mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol. Dis. 2005, 18, 441–449. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequence | Length (bp) | |
|---|---|---|---|
| TNF-α | F: 5′-CCACGCTCTTCTGTCTACTG-3′ | R: 5′-GCTACGGGCTTGTCACTC-3′ | 145 |
| IL-1β | F: 5′-TGTGATGTTCCCATTAGAC-3′ | R: 5′-AATACCACTTGTTGGCTTA-3′ | 131 |
| IL-6 | F: 5′-AGCCACTGCCTTCCCTAC-3′ | R: 5′-TTGCCATTGCACAACTCTT-3′ | 156 |
| iNOS | F: 5′-CACCCAGAAGAGTTACAGC-3′ | R: 5′-GGAGGGAAGGGAGAATAG-3′ | 186 |
| COX-2 | F: 5′-AGAGTCAGTTAGTGGGTAGT-3′ | R: 5′-CTTGTAGTAGGCTTAAACATAG-3′ | 170 |
| β-actin | F: 5′-GTCAGGTCATCACTATCGGCAAT-3′ | R: 5′-AGAGGTCTTTACGGATGTCAACGT-3′ | 147 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Liu, D.; Fu, S. Peiminine Protects Dopaminergic Neurons from Inflammation-Induced Cell Death by Inhibiting the ERK1/2 and NF-κB Signalling Pathways. Int. J. Mol. Sci. 2018, 19, 821. https://doi.org/10.3390/ijms19030821
Chen G, Liu J, Jiang L, Ran X, He D, Li Y, Huang B, Wang W, Liu D, Fu S. Peiminine Protects Dopaminergic Neurons from Inflammation-Induced Cell Death by Inhibiting the ERK1/2 and NF-κB Signalling Pathways. International Journal of Molecular Sciences. 2018; 19(3):821. https://doi.org/10.3390/ijms19030821
Chicago/Turabian StyleChen, Guangxin, Juxiong Liu, Liqiang Jiang, Xin Ran, Dewei He, Yuhang Li, Bingxu Huang, Wei Wang, Dianfeng Liu, and Shoupeng Fu. 2018. "Peiminine Protects Dopaminergic Neurons from Inflammation-Induced Cell Death by Inhibiting the ERK1/2 and NF-κB Signalling Pathways" International Journal of Molecular Sciences 19, no. 3: 821. https://doi.org/10.3390/ijms19030821