How Tyramine β-Hydroxylase Controls the Production of Octopamine, Modulating the Mobility of Beetles
Abstract
:1. Introduction
2. Results
2.1. Molecular Cloning and Sequences Analysis
2.2. Expression Profiles
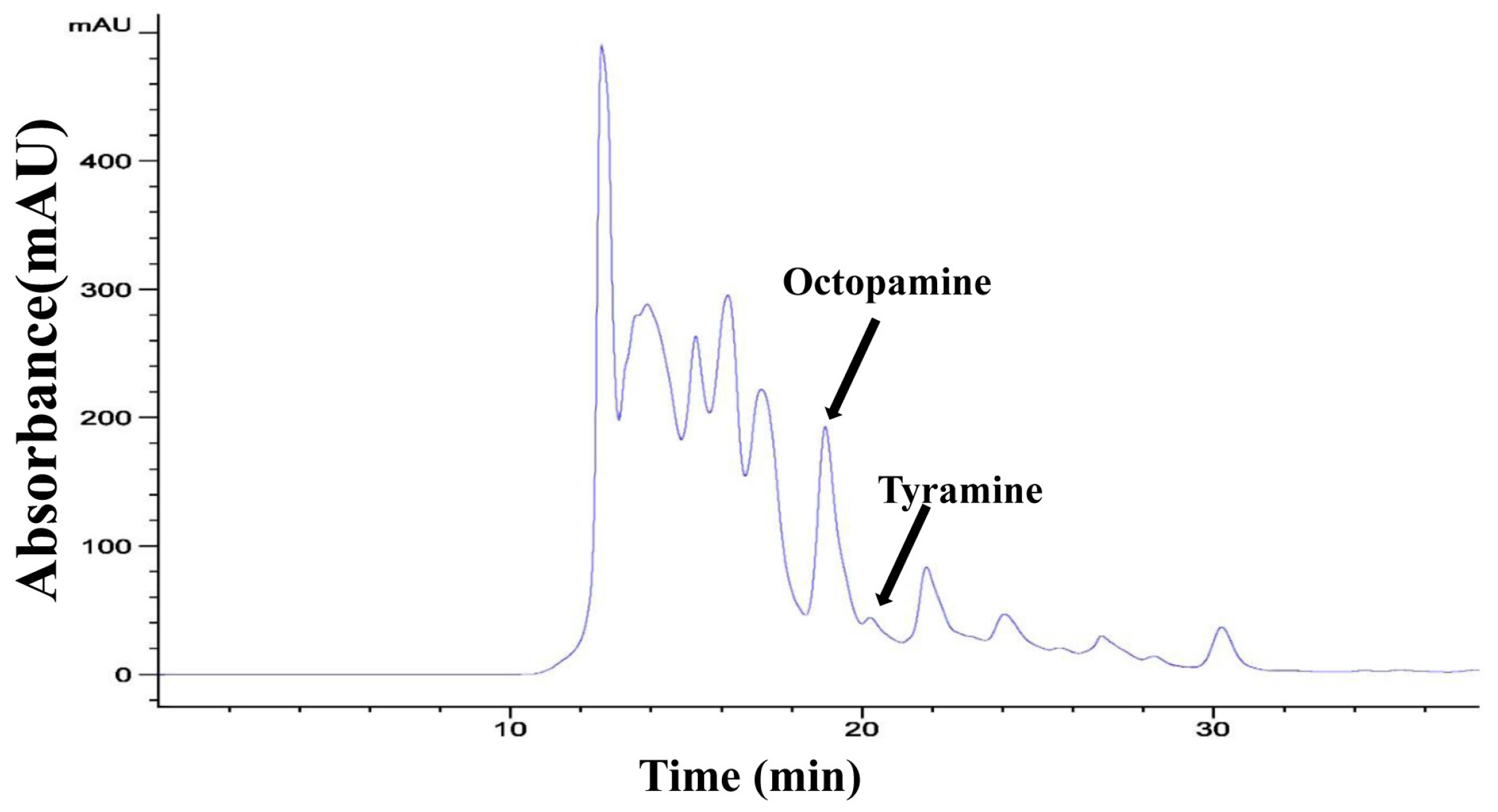
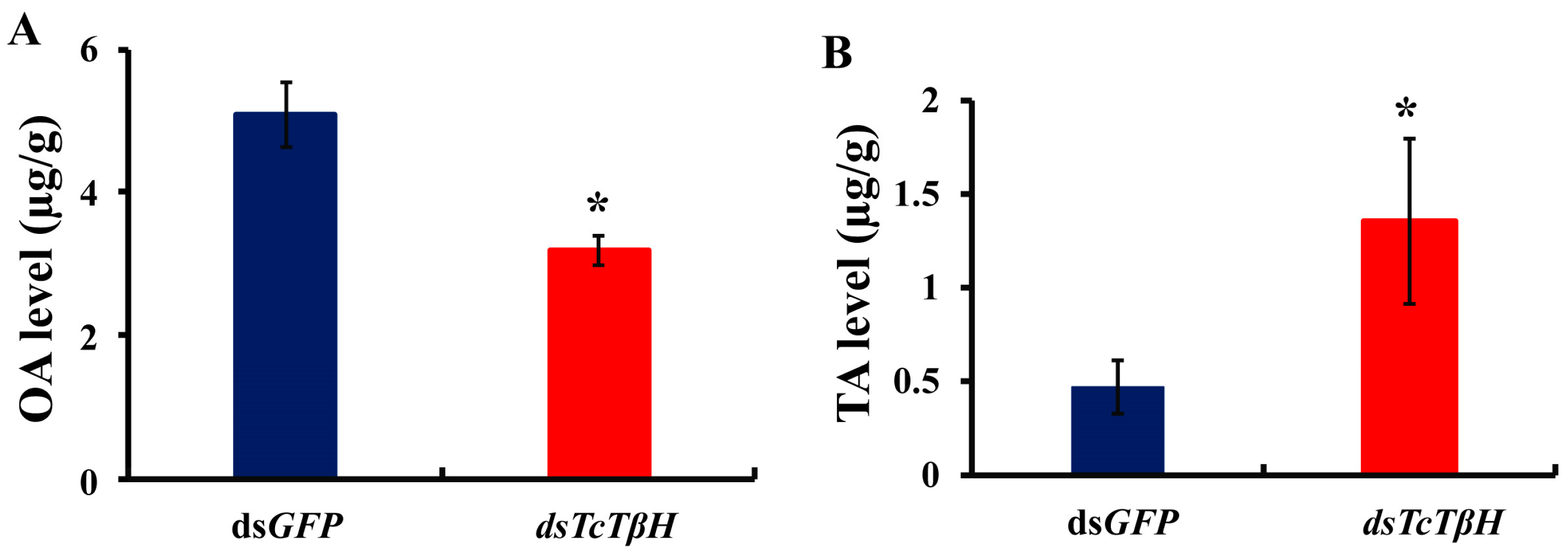
2.3. OA and TA Measurements and Mobility Assay
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Molecular Cloning and Sequences Analysis
4.3. Quantitative Real-Time PCR
4.4. RNA Interference
4.5. Mobility Assay
4.6. OA and TA Detection by High Performance Liquid Chromatography (HPLC)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roeder, T. Tyramine and octopamine: Ruling behavior and metabolism. Ann. Rev. Entomol. 2005, 50, 447–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ng, F.; Lebestky, T.; Grygoruk, A.; Djapri, C.; Lawal, H.O.; Zaveri, H.A.; Mehanzel, F.; Najibi, R.; Seidman, G.; et al. Dispensable, redundant, complementary, and cooperative roles of dopamine, octopamine, and serotonin in Drosophila melanogaster. Genetics 2013, 193, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Guzman, M.G.; Morales-Matos, C.; del Valle Diaz, R.A.; Abramson, C.I.; Giray, T. Dopamine and octopamine influence avoidance learning of honey bees in a place preference assay. PLoS ONE 2011, 6, e25371. [Google Scholar] [CrossRef] [PubMed]
- Burrell, B.D.; Smith, B.H. Modulation of the honey bee (Apis Mellifera) sting response by octopamine. J. Insect Physiol. 1995, 41, 671–680. [Google Scholar] [CrossRef]
- Scheiner, R.; Reim, T.; Sovik, E.; Entler, B.V.; Barron, A.B.; Thamm, M. Learning, gustatory responsiveness and tyramine differences across nurse and forager honeybees. J. Exp. Biol. 2017, 220, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Rillich, J.; Stevenson, P.A. Winning fights induces hyperaggression via the action of the biogenic amine octopamine in Crickets. PLoS ONE 2011, 6, e28891. [Google Scholar] [CrossRef] [PubMed]
- Rillich, J.; Stevenson, P.A.; Pflueger, H.-J. Flight and walking in locusts-cholinergic co-activation, temporal coupling and its modulation by biogenic amines. PLoS ONE 2013, 8, e62899. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, L.; Yang, P.; Ma, Z. Calmodulin as a downstream gene of octopamine-OAR Alpha 1 signalling mediates olfactory attraction in gregarious locusts. Insect Mol. Biol. 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, A.; Hirokado, S.; Tojikubo, R.; Takeya, R.; Taniguchi, E.; Eto, M. Metamorphosis of the red flour beetle, Tribolium freemani Hinton (Coleoptera: Tenebrionidae): Alteration of octopamine content modulates activity of juvenile-hormone esterase, ecdysteroid level, and pupation. Arch. Insect Biochem. Physiol. 1998, 37, 33–46. [Google Scholar] [CrossRef]
- Sujkowski, A.; Ramesh, D.; Brockmann, A.; Wessells, R. Octopamine drives endurance exercise adaptations in Drosophila. Cell Rep. 2017, 21, 1809–1823. [Google Scholar] [CrossRef] [PubMed]
- Adonyeva, N.V.; Burdina, E.V.; Rauschenbach, I.Y.; Menshanov, P.N.; Gruntenko, N.E. Insulin-like receptor substrate gene chico regulates octopamine metabolism in Drosophila melanogaster. Physiol. Entomol. 2017, 42, 85–90. [Google Scholar] [CrossRef]
- Selcho, M.; Pauls, D.; el Jundi, B.; Stocker, R.F.; Thum, A.S. The Role of octopamine and tyramine in Drosophila larval locomotion. J. Comp. Neurol. 2012, 520, 3764–3785. [Google Scholar] [CrossRef] [PubMed]
- Dinges, N.; Morin, V.; Kreim, N.; Southall, T.D.; Roignant, J.-Y. Comprehensive characterization of the complex lola locus reveals a novel role in the octopaminergic pathway via tyramine β-hydroxylase regulation. Cell Rep. 2017, 21, 2911–2925. [Google Scholar] [CrossRef] [PubMed]
- Chatel, A.; Murillo, L.; Bourdin, C.M.; Quinchard, S.; Picard, D.; Legros, C. Characterization of tyramine β-hydroxylase, an enzyme upregulated by stress in Periplaneta americana. J. Mol. Endocrinol. 2013, 50, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lehman, K.K.; Klukas, K.A.; Gilchrist, L.S.; Mesce, K.A. Steroid regulation of octopamine expression during metamorphic development of the moth Manduca sexta. J. Comp. Neurol. 2000, 424, 283–296. [Google Scholar] [CrossRef]
- Lehman, H.K.; Murgiuc, C.M.; Hildebrand, J.G. Characterization and developmental regulation of tyramine-β-hydroxylase in the CNS of the moth, Manduca sexta. Insect Biochem. Mol. Biol. 2000, 30, 377–386. [Google Scholar] [CrossRef]
- Lehman, H.K.; Schulz, D.J.; Barron, A.B.; Wraight, L.; Hardison, C.; Whitney, S.; Takeuchi, H.; Paul, R.K.; Robinson, G.E. Division of labor in the honey bee (Apis mellifera): The role of tyramine β-hydroxylase. J. Exp. Biol. 2006, 209, 2774–2784. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.E.; Soll, D.R.; Wu, C.F. Coordination and modulation of locomotion pattern generators in Drosophila larvae: Effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation. J. Neurosci. 2006, 26, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Zhang, Y.; Zhang, Z.-F.; Tian, H.-G.; Liu, T.-X. Deciphering the function of octopaminergic signaling on wing polyphenism of the pea aphid Acyrthosiphon pisum. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.H.; Hasan, N.; Guo, R.; Ohta, H.; Hirashima, A. Molecular cloning and characterization of a recombinant Bombyx mori tyramine-β-hydroxylase in a silkworm cell line using a baculovirus expression vector system. J. Asia-Pac. Entomol. 2014, 17, 221–227. [Google Scholar] [CrossRef]
- Homberg, U.; Seyfarth, J.; Binkle, U.; Monastirioti, M.; Alkema, M.J. Identification of distinct tyraminergic and octopaminergic neurons innervating the central complex of the desert locust, Schistocerca gregaria. J. Comp. Neurol. 2013, 521, 2025–2041. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.E.; Fontanilla, T.M.; Roncalli, V.; Cieslak, M.C.; Lenz, P.H. Identification and developmental expression of the enzymes responsible for dopamine, histamine, octopamine and serotonin biosynthesis in the copepod crustacean Calanus finmarchicus. Gen. Comp. Endocrinol. 2014, 195, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Monastirioti, M.; Linn, C.E.; White, K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 1996, 16, 3900–3911. [Google Scholar] [PubMed]
- Certel, S.J.; Savella, M.G.; Schlegel, D.C.F.; Kravitz, E.A. Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA 2007, 104, 4706–4711. [Google Scholar] [CrossRef] [PubMed]
- Pflueger, H.-J.; Duch, C. Dynamic neural control of insect muscle metabolism related to motor behavior. Physiology 2011, 26, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.B.; Maleszka, R.; Vander Meer, R.K.; Robinson, G.E. Octopamine modulates honey bee dance behavior. Proc. Natl. Acad. Sci. USA 2007, 104, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, Z.; Nachman, R.J.; Kaczmarek, K.; Zabrocki, J.; Park, Y. Functional characterization of five different PRXamide receptors of the red flour beetle Tribolium castaneum with peptidomimetics and identification of agonists and antagonists. Peptides 2015, 68, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Kim, H.G.; Park, Y. Alternatively spliced orcokinin isoforms and their functions in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 65. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kramer, E. Orientation of walking honeybees in odor fields with small concentration gradients. Physiol. Entomol. 1976, 1, 27–37. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Jiang, H.-B.; Chen, X.-F.; Xiong, Y.; Lu, X.-P.; Pei, Y.-X.; Smagghe, G.; Wang, J.-J. How Tyramine β-Hydroxylase Controls the Production of Octopamine, Modulating the Mobility of Beetles. Int. J. Mol. Sci. 2018, 19, 846. https://doi.org/10.3390/ijms19030846
Xu L, Jiang H-B, Chen X-F, Xiong Y, Lu X-P, Pei Y-X, Smagghe G, Wang J-J. How Tyramine β-Hydroxylase Controls the Production of Octopamine, Modulating the Mobility of Beetles. International Journal of Molecular Sciences. 2018; 19(3):846. https://doi.org/10.3390/ijms19030846
Chicago/Turabian StyleXu, Li, Hong-Bo Jiang, Xiao-Feng Chen, Ying Xiong, Xue-Ping Lu, Yu-Xia Pei, Guy Smagghe, and Jin-Jun Wang. 2018. "How Tyramine β-Hydroxylase Controls the Production of Octopamine, Modulating the Mobility of Beetles" International Journal of Molecular Sciences 19, no. 3: 846. https://doi.org/10.3390/ijms19030846
APA StyleXu, L., Jiang, H.-B., Chen, X.-F., Xiong, Y., Lu, X.-P., Pei, Y.-X., Smagghe, G., & Wang, J.-J. (2018). How Tyramine β-Hydroxylase Controls the Production of Octopamine, Modulating the Mobility of Beetles. International Journal of Molecular Sciences, 19(3), 846. https://doi.org/10.3390/ijms19030846







