Allopregnanolone Alters the Gene Expression Profile of Human Glioblastoma Cells
Abstract
:1. Introduction
2. Results
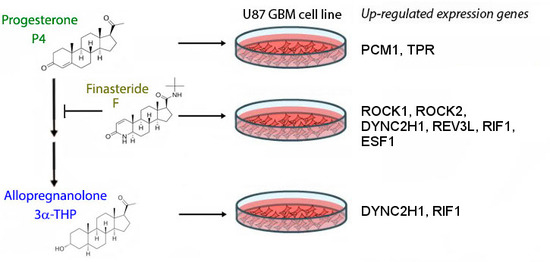
2.1. 3α-THP and F Promote Gene Expression Changes in U87 Cells
2.2. 3α-THP and F Increase the mRNA Levels of Proteins Involved in Several Cellular and Metabolic Processes
2.3. 3α-THP and F Increase the Expression Level of Genes Selected for Validation
2.4. CREB1 and CEBPa Could Mediate 3α-THP-Dependent Transcriptional Effects
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. RNA Extraction and Microarrays
4.3. Validation of Selected Differentially Expressed Genes by RT-qPCR
4.4. Bioinformatic Analysis of Transcription Factor Binding Sites
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncol. 2015, 17, iv1–iv62. [Google Scholar] [CrossRef] [PubMed]
- Schwartzbaum, J.A.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2006, 2, 494–503; [Google Scholar] [CrossRef] [PubMed]
- Piña-Medina, A.G.; Hansberg-Pastor, V.; González-Arenas, A.; Cerbón, M.; Camacho-Arroyo, I. Progesterone promotes cell migration, invasion and cofilin activation in human astrocytoma cells. Steroids 2016, 105, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodríguez, A.; Hansberg-Pastor, V.; Camacho-Arroyo, I. Proliferative and Invasive Effects of Progesterone-Induced Blocking Factor in Human Glioblastoma Cells. Biomed. Res. Int. 2017, 2017, 1295087. [Google Scholar] [CrossRef] [PubMed]
- Faroni, A.; Magnaghi, V. The neurosteroid allopregnanolone modulates specific functions in central and peripheral glial cells. Front. Endocrinol. 2011, 2, 103. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; de Nicola, A.F.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Djebaili, M.; Guo, Q.; Pettus, E.H.; Hoffman, S.W.; Stein, D.G. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma 2005, 22, 106–118. [Google Scholar] [CrossRef] [PubMed]
- VanLandingham, J.W.; Cutler, S.M.; Virmani, S.; Hoffman, S.W.; Covey, D.F.; Krishnan, K.; Hammes, S.R.; Jamnongjit, M.; Stein, D.G. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology 2006, 51, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Johnston, P.B.; Ball, B.G.; Brinton, R.D. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J. Neurosci. 2005, 25, 4706–4718. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-C.; Ping, Y.-F.; Yi, L.; Zhou, Z.-H.; Chen, J.-H.; Yao, X.-H.; Gao, L.; Wang, J.M.; Bian, X.-W. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008, 265, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Karout, M.; Miesch, M.; Geoffroy, P.; Kraft, S.; Hofmann, H.D.; Mensah-Nyagan, A.G.; Kirsch, M. Novel analogs of allopregnanolone show improved efficiency and specificity in neuroprotection and stimulation of proliferation. J. Neurochem. 2016, 139, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.; Uzunova, V.; Matsumoto, K.; Puia, G.; Mienville, J.M.; Costa, E.; Guidotti, A. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology 2000, 39, 440–448. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Membrane progesterone receptors: Evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology 2012, 96, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and ε (mPRδ and mPRε) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ishizuka, Y.; Yamazaki, H.; Shirao, T. Allopregnanolone increases mature excitatory synapses along dendrites via protein kinase A signaling. Neuroscience 2015, 305, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Langmade, S.J.; Gale, S.E.; Frolov, A.; Mohri, I.; Suzuki, K.; Mellon, S.H.; Walkley, S.U.; Covey, D.F.; Schaffer, J.E.; Ory, D.S. Pregnane X receptor (PXR) activation: A mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA 2006, 103, 13807–13812. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sánchez, C.J.; Hansberg-Pastor, V.; Salido-Guadarrama, I.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Allopregnanolone promotes proliferation and differential gene expression in human glioblastoma cells. Steroids 2017. [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- GEO Accession Viewer. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi (accessed on 24 February 2018).
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Scharpf, R.B.; Bravo, H.C.; Simcha, D.; Langmead, B.; Johnson, W.E.; Geman, D.; Baggerly, K.; Irizarry, R.A. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 2010, 11, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008, 29, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, O.T.; González-García, T.K.; Camacho-Arroyo, I. Progesterone receptor and SRC-1 participate in the regulation of VEGF, EGFR and Cyclin D1 expression in human astrocytoma cell lines. J. Steroid Biochem. Mol. Biol. 2012, 132, 127–134. [Google Scholar] [CrossRef] [PubMed]
- González-Arenas, A.; Cabrera-Wrooman, A.; Díaz, N.; González-García, T.; Salido-Guadarrama, I.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Progesterone receptor subcellular localization and gene expression profile in human astrocytoma cells are modified by progesterone. Nucl. Recept. Res. 2014, 1, 1–10. [Google Scholar] [CrossRef]
- González-Arenas, A.; Peña-Ortiz, M.Á.; Hansberg-Pastor, V.; Marquina-Sánchez, B.; Baranda-Ávila, N.; Nava-Castro, K.; Cabrera-Wrooman, A.; González-Jorge, J.; Camacho-Arroyo, I. PKCα and PKCδ Activation Regulates Transcriptional Activity and Degradation of Progesterone Receptor in Human Astrocytoma Cells. Endocrinology 2015, 156, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Faivre, E.J.; Daniel, A.R.; Hillard, C.J.; Lange, C.A. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol. Endocrinol. 2008, 22, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.A.; Shen, T.; Horwitz, K.B. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 2000, 97, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Reul, J.M.H.M.; Trapp, T.; Steensel, B.v.; Wetzel, C.; Damm, K.; Zieglgänsberger, W.; Holsboer, F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron 1993, 11, 523–530. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, C.; Wu, H.; Xu, Z.; Wei, L.; Zhao, R.; Jin, D. Finasteride reduces microvessel density and expression of vascular endothelial growth factor in renal tissue of diabetic rats. Am. J. Med. Sci. 2015, 349, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Guterman, P.; Langlois, V.S.; Hodgkinson, K.; Pauli, B.D.; Cooke, G.M.; Wade, M.G.; Trudeau, V.L. The aromatase inhibitor fadrozole and the 5-reductase inhibitor finasteride affect gonadal differentiation and gene expression in the frog Silurana tropicalis. Sex Dev. 2009, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.; Seyam, R.M.; Brock, G.B. Reduction of Ventral Prostate Weight by Finasteride Is Associated with Suppression of Insulin-like Growth Factor I (IGF-I) and IGF-I Receptor Genes and with an Increase in IGF Binding Protein 3 Reduction of Ventral Prostate Weight by Finasteride Is Asso. Cancer Res. 1998, 58, 215–218. [Google Scholar] [PubMed]
- Chhipa, R.R.; Halim, D.; Cheng, J.; Zhang, H.Y.; Mohler, J.L.; Ip, C.; Wu, Y. The direct inhibitory effect of dutasteride or finasteride on androgen receptor activity is cell line specific. Prostate 2013, 73, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Godoy, A.; Azzouni, F.; Wilton, J.H.; Ip, C.; Mohler, J.L. Prostate cancer cells differ in testosterone accumulation, dihydrotestosterone conversion, and androgen receptor signaling response to steroid 5α-reductase inhibitors. Prostate 2013, 73, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv. Urol. 2012, 2012, 530121. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.P.; Muzia, D.; Hu, J.; Szwajcer, D.; Hill, S.A.; Seachrist, J.L. The 4-pregnene and 5α-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res. 2000, 60, 936–943. [Google Scholar] [PubMed]
- Beck, C.A.; Wolfe, M.; Murphy, L.D.; Wiebe, J.P. Acute, nongenomic actions of the neuroactive gonadal steroid, 3α-hydroxy-4-pregnen-20-one (3αHP), on FSH release in perifused rat anterior pituitary cells. Endocrine 1997, 6, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Chang, Z.-F.; Lee, H.-H. Immunohistochemical evaluation of ROCK activation in invasive breast cancer. BMC Cancer 2015, 15, 943. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Kano, Y.; Noda, Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. J. R. Soc. Interface 2011, 8, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, W.-D.; Tan, Z.-M.; Fang, W.-G.; Zhu, L.; Chen, Y.-H. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 2006, 580, 4252–4260. [Google Scholar] [CrossRef] [PubMed]
- Qin, E.Y.; Cooper, D.D.; Abbott, K.L.; Lennon, J.; Nagaraja, S.; Mackay, A.; Jones, C.; Vogel, H.; Jackson, P.K.; Monje, M. Neural Precursor-Derived Pleiotrophin Mediates Subventricular Zone Invasion by Glioma. Cell 2017, 170, 845–859e19. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Guo, X.; Gao, X.; Xue, H.; Zhang, J.; Guo, X.; Qiu, W.; Zhang, P.; Li, G. Macrophage migration inhibitory factor enhances autophagy by regulating ROCK1 activity and contributes to the escape of dendritic cell surveillance in glioblastoma. Int. J. Oncol. 2016, 49, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Melfi, S.; Montt Guevara, M.M.; Bonalume, V.; Ruscica, M.; Colciago, A.; Simoncini, T.; Magnaghi, V. Src and phospho-FAK kinases are activated by allopregnanolone promoting Schwann cell motility. J. Neurochem. 2017, 141, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Ishii, A.; Kanoh, Y.; Oda, M.; Nishito, Y.; Masai, H. RIF1 regulates the replication timing domains on the human genome. EMBO J. 2012, 31, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, A.; Chen, L.; Zhong, X.; Liao, J.; Gao, M.; Cai, M.; Lee, D.H.; Li, J.; Chowdhury, D.; et al. Human RIF1 encodes an anti-apoptotic factor required for DNA repair. Carcinogenesis 2009, 30, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, W.; Lu, Y.; Li, H.; Xiang, W.; Chen, Z.; He, M.; Zhao, L.; Sun, X.; Lei, B.; et al. Expression of dynein, cytoplasmic 2, heavy chain 1 (DHC2) associated with glioblastoma cell resistance to temozolomide. Sci. Rep. 2016, 6, 28948. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Cosmes, P.; Vázquez-Martínez, E.R.; Cerbón, M.; Camacho-Arroyo, I. Membrane progesterone receptors in reproduction and cancer. Mol. Cell. Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Daniel, P.; Filiz, G.; Brown, D.V.; Hollande, F.; Gonzales, M.; D’Abaco, G.; Papalexis, N.; Phillips, W.A.; Malaterre, J.; Ramsay, R.G.; et al. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis 2014, 3, e108. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.; McCorkle, S.R.; Cha-Molstad, H.; Dwyer, J.M.; Yochum, G.S.; Boss, J.M.; McWeeney, S.; Dunn, J.J.; Mandel, G.; Goodman, R.H. Defining the CREB regulon: A genome-wide analysis of transcription factor regulatory regions. Cell 2004, 119, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V.; Parducz, A.; Frasca, A.; Ballabio, M.; Procacci, P.; Racagni, G.; Bonanno, G.; Fumagalli, F. GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J. Neurochem. 2010, 112, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Routes, J.M.; Colton, L.A.; Ryan, S.; Klemm, D.J. CREB (cAMP response element binding protein) and C/EBPalpha (CCAAT/enhancer binding protein) are required for the superstimulation of phosphoenolpyruvate carboxykinase gene transcription by adenoviral E1a and cAMP. Biochem. J. 2000, 352 Pt 2, 335–342. [Google Scholar] [CrossRef]
- Zhang, J.W.; Klemm, D.J.; Vinson, C.; Lane, M.D. Role of CREB in Transcriptional Regulation of CCAAT/Enhancer-binding Protein β Gene during Adipogenesis. J. Biol. Chem. 2004, 279, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Katara, R.; Mir, R.A.; Shukla, A.A.; Tiwari, A.; Singh, N.; Chauhan, S.S. Wild type p53-dependent transcriptional upregulation of cathepsin L expression is mediated by C/EBPa in human glioblastoma cells. Biol. Chem. 2010, 391, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Hariparsad, N.; Chu, X.; Yabut, J.; Labhart, P.; Hartley, D.P.; Dai, X.; Evers, R. Identification of pregnane-X receptor target genes and coactivator and corepressor binding to promoter elements in human hepatocytes. Nucleic Acids Res. 2009, 37, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: http://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 31 August 2017).
- Thomas, P.D. PANTHER: A Library of Protein Families and Subfamilies Indexed by Function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Dong, Q.; Muruganujan, A.; Gaudet, P.; Lewis, S.; Thomas, P.D. PANTHER version 7: Improved phylogenetic trees, orthologs and collaboration with the Gene Ontology Consortium. Nucleic Acids Res. 2009, 38, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Kejariwal, A.; Guo, N.; Mi, H.; Campbell, M.J.; Muruganujan, A.; Lazareva-Ulitsky, B. Applications for protein sequence-function evolution data: MRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 2006, 34, 645–650. [Google Scholar] [CrossRef]
- PANTHER—Gene List Analysis. Available online: http://pantherdb.org/ (accessed on 24 February 2018).
- DAVID Functional Annotation Bioinformatics Microarray Analysis. Available online: https://david.ncifcrf.gov/ (accessed on 24 February 2018).
- BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 24 February 2018).
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2003–2007. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Human Genome Resources at NCBI. Available online: https://www.ncbi.nlm.nih.gov/genome/guide/human/ (accessed on 24 February 2018).
- Yates, A.; Akanni, W.; Amode, M.R.; Barrell, D.; Billis, K.; Carvalho-Silva, D.; Cummins, C.; Clapham, P.; Fitzgerald, S.; Gil, L.; et al. Ensembl 2016. Nucleic Acids Res. 2016, 44, D710–D716. [Google Scholar] [CrossRef] [PubMed]
- Dreos, R.; Ambrosini, G.; Périer, R.C.; Bucher, P. The Eukaryotic Promoter Database: Expansion of EPDNew and new promoter analysis tools. Nucleic Acids Res. 2015, 43, D92–D96. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Fornes, O.; Stigliani, A.; Gheorghe, M.; Castro-Mondragon, J.A.; van der Lee, R.; Bessy, A.; Chèneby, J.; Kulkarni, S.R.; Tan, G.; et al. JASPAR 2018: Update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018, 46, D260–D266. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M. The Human Genome Browser at UCSC W. J. Med. Chem. 1976, 19, 1228–1231. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Varlamov, A.; Vaskin, Y.; Efremov, I.; German Grehov, O.G.; Kandrov, D.; Rasputin, K.; Syabro, M.; et al. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Matys, V. TRANSFAC(R) and its module TRANSCompel(R): Transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006, 34, D108–D110. [Google Scholar] [CrossRef] [PubMed]




| Gene Full Name | Treatment vs. V (Fc) | Treatment vs. V (p-Value) | Ontogeny Categories | ||
|---|---|---|---|---|---|
| 3α-THP | F | 3α-THP | F | ||
| ESF1 nucleolar pre-rRNA processing protein homolog (ESF1) | 3.07 | 2.01 | 0.0103 | 0.044 | ND |
| Translocated promoter region, nuclear basket protein (TPR) | 2.75 | - | 0.010 | - |
|
| Replication timing regulatory factor 1 (RIF1) | 2.69 | - | 0.015 | - | ND |
| RAD50 double-strand break repair protein (RAD50) | 2.31 | 2.16 | 0.010 | 0.022 |
|
| Rho-associated coiled-coil containing protein kinase 1 (ROCK1) | 1.94 | 2.04 | 0.009 | 0.018 |
|
| Rho-associated coiled-coil containing protein kinase 2 (ROCK2) | 1.94 | 1.8 | 0.041 | 0.034 |
|
| REV3-like, DNA directed polymerase zeta catalytic subunit (REV3L) | 1.86 | - | 0.014 | - |
|
| Pericentriolar material 1 protein (PCM1) | 1.68 | 1.54 | 0.037 | 0.044 |
|
| Dynein cytoplasmic 2 heavy chain 1 (DYNC2H1) | 1.64 | 1.55 | 0.001 | 0.023 |
|
| Coiled-coil domain containing 91, P56 protein (CCDC91) | 1.63 | - | 0.04 | - | ND |
| Gene | Primer Sequence 5′→3′ | Amplified Fragment (bp) |
|---|---|---|
| ESF1 | FW: GCTCCTCGTGCTGATGAGATTA | 176 |
| RV: TGCTCTTCCTTCATCCTCTCCT | ||
| PCM1 | FW: TCAAGACAAGAAAAGCGTCTGC | 180 |
| RV: GGGCTGAATGTCTGTTCCTACT | ||
| TPR | FW: TTTGGCACAGTTTCGGCTAC | 164 |
| RV: TCTTCCTCAGTTCCTACAGGTG | ||
| RIF1 | FW: TAATAAGGTTCGCCGTGTCTCC | 177 |
| RV: CCTTTGGCTGAAGTGGTATTATGC | ||
| REV3L | FW: TGAGAAATGAGGTGGCTCTAAC | 168 |
| RV: CACGGACACGGCTAACATAA | ||
| RAD50 | FW: GCCTCACTCATCATTCGCCT | 168 |
| RV: AAGCTGGAAGTTACGCTGCT | ||
| ROCK1 | FW: ATGGAACCAGTACAACAAGCTGA | 159 |
| RV: GCATCTTCGACACTCTAGGGC | ||
| ROCK2 | FW: GAAGAGCAGCAGAAGTGGGT | 170 |
| RV: GGCAGTTAGCTAGGTTTGTTTGG | ||
| CCDC91 | FW: AAGTCAGGAAACTGTTAAGGCAG | 152 |
| RV: ACAGGCTTCTTTGGCGGAT | ||
| DYNC2H1 | FW: GCTTGGCGGAGCAGATTAAA | 159 |
| RV: CCAGGATGCCCGATTCAGTAT | ||
| 18S | FW: AGTGAAACTGCAATGGCTC | 167 |
| RV: CTGACCGGGTTGGTTTTGAT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Sánchez, C.J.; Del Moral-Morales, A.; Hernández-Vega, A.M.; Hansberg-Pastor, V.; Salido-Guadarrama, I.; Rodríguez-Dorantes, M.; Camacho-Arroyo, I. Allopregnanolone Alters the Gene Expression Profile of Human Glioblastoma Cells. Int. J. Mol. Sci. 2018, 19, 864. https://doi.org/10.3390/ijms19030864
Zamora-Sánchez CJ, Del Moral-Morales A, Hernández-Vega AM, Hansberg-Pastor V, Salido-Guadarrama I, Rodríguez-Dorantes M, Camacho-Arroyo I. Allopregnanolone Alters the Gene Expression Profile of Human Glioblastoma Cells. International Journal of Molecular Sciences. 2018; 19(3):864. https://doi.org/10.3390/ijms19030864
Chicago/Turabian StyleZamora-Sánchez, Carmen J., Aylin Del Moral-Morales, Ana M. Hernández-Vega, Valeria Hansberg-Pastor, Ivan Salido-Guadarrama, Mauricio Rodríguez-Dorantes, and Ignacio Camacho-Arroyo. 2018. "Allopregnanolone Alters the Gene Expression Profile of Human Glioblastoma Cells" International Journal of Molecular Sciences 19, no. 3: 864. https://doi.org/10.3390/ijms19030864