Distinct Properties of Human M-CSF and GM-CSF Monocyte-Derived Macrophages to Simulate Pathological Lung Conditions In Vitro: Application to Systemic and Inflammatory Disorders with Pulmonary Involvement
Abstract
:1. Introduction
2. Results
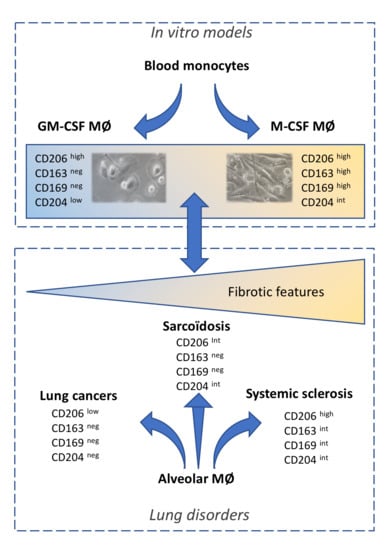
2.1. Phenotype of M-MDMs, GM-MDMs and AM
2.2. Phenotypic Differences among AM of Patients Suffering from Lung Neoplasia, Sarcoidosis and SSc Associated ILD
2.3. Co-Expression of CD206, CD163 and CD169 in Blood-MDMs and AM
2.4. Phenotypic Variations of Blood-MDMs from Healthy Donors and SSc Patients, and Comparisons with AM in SSc-Associated Lung Disease
2.5. Secretion Levels of the Pro-Inflammatory Cytokine IL-6 and the Pro-Fibrotic Chemokine CCL18
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Monocyte Derived Macrophages
4.2. Characteristics of Patients
4.3. Bronchoalveolar Lavages (BALs)
4.4. Flow Cytometry Analysis
4.5. Quantification of Cytokine and Chemokine Levels
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Gordon, S.; Hume, D.A.; Mowat, A.M.; Randolph, G.J. Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol. 2010, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Modolell, M.; La Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: Granulomatous pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Baran, C.P.; Opalek, J.M.; McMaken, S.; Newland, C.A.; O’Brien, J.M., Jr.; Hunter, M.G.; Bringardner, B.D.; Monick, M.M.; Brigstock, D.R.; Stromberg, P.C.; et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.M.; Taroni, J.; Martyanov, V.; Wood, T.A.; Greene, C.S.; Pioli, P.A.; Hinchcliff, M.E.; Whitfield, M.L. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLoS Comput. Biol. 2015, 11, e1004005. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.Y. Lung inflammation and fibrosis: An alveolar macrophage-centered perspective from the 1970s to 1980s. Am. J. Respir. Crit. Care Med. 2005, 171, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Stifano, G.; Christmann, R.B. Macrophage Involvement in Systemic Sclerosis: Do We Need More Evidence? Curr. Rheumatol. Rep. 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Coiffier, G.; de Carlan, M.; Droitcourt, C.; Ballerie, A.; Cazalets, C.; Perdriger, A.; Jego, P. Combination of capillaroscopic and ultrasonographic evaluations in systemic sclerosis: Results of a cross-sectional study. Arthritis Care Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; de Waal-Malefyt, R.; Ottenhoff, T.H. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, R.; Schubert, W.; Gunther, L.; Kress, Y.; Macaluso, F.; Pollard, J.W.; McMurray, D.N.; Bloom, B.R. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity 1999, 10, 641–650. [Google Scholar] [CrossRef]
- Martinez-Moczygemba, M.; Huston, D.P. Immune dysregulation in the pathogenesis of pulmonary alveolar proteinosis. Curr. Allergy Asthma Rep. 2010, 10, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Ballerie, A.; Nimubona, S.; Meunier, C.; Gutierrez, F.L.; Desrues, B.; Delaval, P.; Jouneau, S. Association of pulmonary alveolar proteinosis and fibrosis: Patient with GATA2 deficiency. Eur. Respir. J. 2016, 48, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Higashi-Kuwata, N.; Jinnin, M.; Makino, T.; Fukushima, S.; Inoue, Y.; Muchemwa, F.C.; Yonemura, Y.; Komohara, Y.; Takeya, M.; Mitsuya, H.; et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res. Ther. 2010, 12, R128. [Google Scholar] [CrossRef] [PubMed]
- York, M.R.; Nagai, T.; Mangini, A.J.; Lemaire, R.; van Seventer, J.M.; Lafyatis, R. A macrophage marker, Siglec-1, is increased on circu- lating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007, 56, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Christmann, R.B.; Hayes, E.; Pendergrass, S.; Padilla, C.; Farina, G.; Affandi, A.J.; Whitfield, M.L.; Farber, H.W.; Lafyatis, R. Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum. 2011, 63, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Christmann, R.B.; Sampaio-Barros, P.; Stifano, G.; Borges, C.L.; Carvalho, C.R.; Kairalla, R.; Parra, E.R.; Spira, A.; Simms, R.; Capellozzi, V.L.; et al. Association of Interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol. 2014, 66, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Beyer, C.; Palumbo-Zerr, K.; Zhang, Y.; Ramming, A.; Distler, A.; Gelse, K.; Distler, O.; Schett, G.; Wollin, L.; et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Maier, C.; Zhang, Y.; Soare, A.; Dees, C.; Beyer, C.; Harre, U.; Chen, C.W.; Distler, O.; Schett, G.; et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef] [PubMed]
- Beamer, C.A.; Holian, A. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L186–L195. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Freeman, M.; Rohrer, L.; Zabrecky, J.; Matsudaira, P.; Krieger, M. Type I macrophage scavenger receptor contains-helical and collagen-like coiled coils. Nature 1990, 343, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Platt, N.; Gordon, S. Is the class A macrophage scavenger receptor (SR-A) multifunctional?—The mouse’s tale. J. Clin. Investig. 2001, 108, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Schupp, J.; Jäger, B.; Schmid, M.; Zissel, G.; Müller-Quernheim, J.; Prasse, A. Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophageactivation. PLoS ONE 2013, 8, e81382. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.C.; Strek, M.E. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann. Am. Thorac. Soc. 2013, 10, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Valeyre, D.; Prasse, A.; Nunes, H.; Uzunhan, Y.; Brillet, P.Y.; Müller-Quernheim, J. Sarcoidosis. Lancet 2014, 383, 1155–1167. [Google Scholar] [CrossRef]
- Wikén, M.; Idali, F.; Al Hayja, M.A.; Grunewald, J.; Eklund, A.; Wahlström, J. No evidence of altered alveolar macrophage polarization, but reduced expression of TLR2, in bronchoalveolar lavage cells in sarcoidosis. Respir. Res. 2010, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J.; Becker, M.; Günther, J.; Müller-Quernheim, J.; Riemekasten, G.; Prasse, A. Serum CCL18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur. Respir. J. 2014, 43, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, J.; Kill, A.; Mattat, K.; Dragun, D.; Siegert, E.; Günther, J.; Riemekasten, G. Monocytic Angiotensin and Endothelin Receptor Imbalance Modulate Secretion of the Profibrotic Chemokine Ligand 18. J. Rheumatol. 2016, 43, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Taroni, J.N.; Greene, C.S.; Martyanov, V.; Wood, T.A.; Christmann, R.B.; Farber, H.W.; Lafyatis, R.A.; Denton, C.P.; Hinchcliff, M.E.; Pioli, P.A.; et al. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Lecureur, V.; Roussel, M.; Sunnaram, B.L.; Ballerie, A.; Coiffier, G.; Jouneau, S.; Fardel, O.; Fest, T.; Jégo, P. CD16-positive circulating monocytes and fibrotic manifestations of systemic sclerosis. Clin. Rheumatol. 2017, 36, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sugimoto, C.; Arainga, M.; Alvarez, X.; Didier, E.S.; Kuroda, M.J. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: Implications for understanding lung disease in humans. J. Immunol. 2014, 192, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J. Systemic sclerosis: STAT3—A key integrator of profibrotic signalling. Nat. Rev. Rheumatol. 2017, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [PubMed]
- Fu, X.L.; Duan, W.; Su, C.Y.; Mao, F.Y.; Lv, Y.P.; Teng, Y.S.; Yu, P.W.; Zhuang, Y.; Zhao, Y.L. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol. Immunother. 2017, 66, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Denton, C.P.; Jahreis, A.; Van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet 2016, 387, 2630–2640. [Google Scholar] [CrossRef]
- Khan, K.; Xu, S.; Nihtyanova, S.; Derrett-Smith, E.; Abraham, D.; Denton, C.P.; Ong, V.H. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann. Rheum. Dis. 2012, 71, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Bolster, M.B.; Ludwicka, A.; Sutherland, S.E.; Strange, C.; Silver, R.M. Cytokine concentrations in bronchoalveolar lavage fluid of patients with systemic sclerosis. Arthritis Rheum. 1997, 40, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.C.; Achuthan, A.; Fleetwood, A.J.; Dinh, H.; Roiniotis, J.; Scholz, G.M.; Chang, M.W.; Beckman, S.K.; Cook, A.D.; Hamilton, J.A. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 2012, 188, 5752–5765. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; So, E.C.; Strome, S.E.; Zhang, X. Impact of Detachment Methods on M2 Macrophage Phenotype and Function. J. Immunol. Methods 2015, 426, 56–61. [Google Scholar] [CrossRef] [PubMed]






| Patient’s Characteristics | ILD-SSc | Sarcoidosis | Neoplasia | All |
|---|---|---|---|---|
| n | 5 | 5 | 6 | 16 |
| Age + SD (in year) | 62.6 + 8.5 | 54.2 + 7.4 | 55.2 + 9.9 | 57.2 + 9.1 |
| number of women (% of women) | 3 (60) | 0 (0) | 2 (33) | 5 (31) |
| Number of Current smoker or ex-smoker (in %) | 2 (40) | 2 (40) | 6 (100) | 10 (67) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lescoat, A.; Ballerie, A.; Augagneur, Y.; Morzadec, C.; Vernhet, L.; Fardel, O.; Jégo, P.; Jouneau, S.; Lecureur, V. Distinct Properties of Human M-CSF and GM-CSF Monocyte-Derived Macrophages to Simulate Pathological Lung Conditions In Vitro: Application to Systemic and Inflammatory Disorders with Pulmonary Involvement. Int. J. Mol. Sci. 2018, 19, 894. https://doi.org/10.3390/ijms19030894
Lescoat A, Ballerie A, Augagneur Y, Morzadec C, Vernhet L, Fardel O, Jégo P, Jouneau S, Lecureur V. Distinct Properties of Human M-CSF and GM-CSF Monocyte-Derived Macrophages to Simulate Pathological Lung Conditions In Vitro: Application to Systemic and Inflammatory Disorders with Pulmonary Involvement. International Journal of Molecular Sciences. 2018; 19(3):894. https://doi.org/10.3390/ijms19030894
Chicago/Turabian StyleLescoat, Alain, Alice Ballerie, Yu Augagneur, Claudie Morzadec, Laurent Vernhet, Olivier Fardel, Patrick Jégo, Stéphane Jouneau, and Valérie Lecureur. 2018. "Distinct Properties of Human M-CSF and GM-CSF Monocyte-Derived Macrophages to Simulate Pathological Lung Conditions In Vitro: Application to Systemic and Inflammatory Disorders with Pulmonary Involvement" International Journal of Molecular Sciences 19, no. 3: 894. https://doi.org/10.3390/ijms19030894