Instillation of Sericin Enhances Corneal Wound Healing through the ERK Pathway in Rat Debrided Corneal Epithelium
Abstract
:1. Introduction
2. Results
2.1. Effect of Sericin on the Akt and ERK Signalings in HCE-T Cells
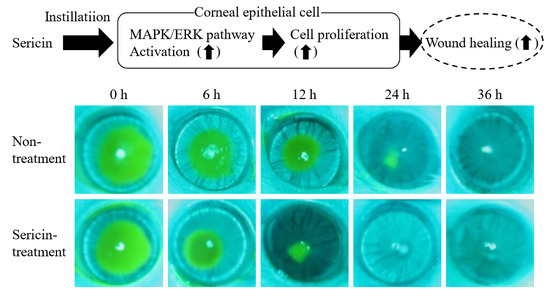
2.2. Enhancement of the Corneal Wound Healing by Sericin via ERK1/2
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Cell Culture
4.4. Western Blot Analysis
4.5. Analysis of Corneal Wound Healing
4.6. Safety Evaluation of Sericin in the Cornea
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| Abs | Absorbance |
| HCE-T cells | Human corneal epithelial cell line |
| NGF | Nerve growth factor |
| pp38 | Phosphorylation of p38 |
| pAkt | Phosphorylation of Akt |
| pERK | Phosphorylation of ERK |
| pJNK | Phosphorylation of JNK |
| U0126 | ERK inhibitor |
References
- Li, Y.; Inoue, T.; Takamatsu, F.; Kobayashi, T.; Shiraishi, A.; Maeda, N.; Ohashi, Y.; Nishida, K. Differences between niche cells and limbal stromal cells in maintenance of corneal limbal stem cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, M.; Greene, C.; Green, C.R. Wound healing in the eye: Therapeutic prospects. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-healing studies in cornea and skin: Parallels, differences and opportunities. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2012, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, L.; Chen, L.; Mao, X.; Ren, F. Antioxidant activities of silk sericin from silkworm Bombyx mori. J. Food Biochem. 2009, 33, 74–88. [Google Scholar] [CrossRef]
- Tamada, Y.; Sano, M.; Niwa, K.; Imai, T.; Yoshino, G. Sulfation of silk sericin and anticoagulant activity of sulfated sericin. J. Biomater. Sci. Polym. Ed. 2004, 15, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Acharya, C.; Bindu, P.C.; Kundu, S.C. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 2008, 41, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Sasaki, M.; Yanagihara, K.; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng. 2005, 100, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Ma, Y.; Xia, Y.Y.; Shen, W.D.; Mao, J.P.; Xue, R.Y. Silk sericin-insulin bioconjugates: Synthesis, characterization and biological activity. J. Control. Release 2006, 115, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Priya, A.S.; Kundu, S.C. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009, 5, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Tsubouchi, K.; Igarashi, Y.; Takasu, Y.; Yamada, H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci. Biotechnol. Biochem. 2005, 69, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effect of sericin on corneal wound healing in rat debrided corneal epithelium. Biol. Pharm. Bull. 2009, 32, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effects of sericin on corneal wound healing in Otsuka long-evans Tokushima Fatty rats as a model of human type 2 diabetes. Biol. Pharm. Bull. 2009, 32, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. Therapeutic effects of sericin on diabetic keratopathy in OLETF rat. World J. Diabetes 2013, 4, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, E52. [Google Scholar] [CrossRef] [PubMed]
- Stupack, D.G.; Cho, S.Y.; Klemke, R.L. molecular signaling mechanisms of cell migration and invasion. Immunol. Res. 2000, 21, 83–88. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers, Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [PubMed]
- Huang, C.; Rajfur, Z.; Borchers, C.; Schaller, M.D.; Jacobson, K. JNK phosphorylates paxillin and regulates cell migration. Nature 2003, 424, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Fitsialos, G.; Chassot, A.A.; Turchi, L.; Dayem, M.A.; LeBrigand, K.; Moreilhon, C.; Meneguzzi, G.; Buscà, R.; Mari, B.; Barbry, P.; et al. Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK1/2, p38, and phosphatidylinositol 3-kinase signaling pathways. J. Biol. Chem. 2007, 282, 15090–15102. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.P.; Li, Y.; Ljubimov, A.V.; Yu, F.S. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 2009, 58, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Qian, T.; Le, Q.; Sun, X.; Wu, J.; Chen, J.; Yu, X.; Xu, J. NGF promotes cell cycle progression by regulating D-type cyclins via PI3K/Akt and MAPK/Erk activation in human corneal epithelial cells. Mol. Vis. 2012, 18, 758–764. [Google Scholar] [PubMed]
- Lake, D.; Corrêa, S.A.; Müller, J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell. Mol. Life Sci. 2016, 73, 4397–4413. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Yamada, M.; Yoshikawa, K.; Iino, M. Ganka-Kaigyoui Notameno Gimon·Nanmon Kaiketusaku; Shindan to Chiryosha Co.: Tokyo, Japan, 2006; pp. 216–217. (In Japanease) [Google Scholar]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Liu, C.Y.; Kao, W.W. Corneal epithelial wound healing. Prog. Mol. Biol. Transl. Sci. 2015, 134, 61–71. [Google Scholar] [PubMed]
- Yoon, J.J.; Ismail, S.; Sherwin, T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J. Stem Cells 2014, 6, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Ní, D.S.; Koppen, C.; Tassignon, M.J.; Zakaria, N. Limbal stem cell deficiency: Current treatment options and emerging therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef] [PubMed]
- Manes, S.; Mira, E.; Gomez-Mouton, C.; Lacalle, R.A.; Martonez-A, C. Cells on the move: A dialogue between polarization and motility. IUBMB Life 2000, 49, 89–96. [Google Scholar] [PubMed]
- Zieske, J.D.; Gipson, I.K. Agents that Affect Corneal Wound Healing: Modulation of Structure and Function; Albert, D.M., Jakobiec, F.A., Eds.; Saunders Press: Philadelphia, PA, USA, 1994; pp. 1093–1109. [Google Scholar]
- Lu, Z.Y.; Chen, W.C.; Li, H.Y.; Li, L.; Zhang, H.; Pang, Y.; Xiao, Z.F.; Xiao, H.W.; Xiao, Y. TNF-α enhances vascular cell adhesion molecule-1 expression in human bone marrow mesenchymal stem cells via the NF-κB, ERK and JNK signaling pathways. Mol. Med. Rep. 2016, 14, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.; Dergham, P.; Escoll, P.; de-la-Hera, A.; D’Onofrio, P.M.; Magharious, M.M.; Koeberle, P.D.; Frade, J.M.; Saragovi, H.U. Neuronal injury external to the retina rapidly activates retinal glia, followed by elevation of markers for cell cycle re-entry and death in retinal ganglion cells. PLoS ONE 2014, 9, e101349. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xie, X.; Lei, Y.; An, G.; He, L.; Lv, X. Ocular albinism type 1-induced melanoma cell migration is mediated through the RAS/RAF/MEK/ERK signaling pathway. Mol. Med. Rep. 2014, 10, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Li, J.; Rao, W.; Liu, W.; Chen, X.; Su, N.; Wang, Y.; Fei, Z. Upregulation of homer1a promoted retinal ganglion cell survival after retinal ischemia and reperfusion via interacting with Erk pathway. Cell. Mol. Neurobiol. 2015, 35, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Parsons, J.T.; Horwitz, A.F. Adhesion assembly, disassembly and turnover in migrating cells—Over and over and over again. Nat. Cell Biol. 2002, 4, E97–E100. [Google Scholar] [CrossRef] [PubMed]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing. Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar] [PubMed]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]







| Treatment | kH (×10−2/h) |
|---|---|
| Control | 4.81 ± 0.80 *2,3 |
| Vehicle | 4.25 ± 0.81 *2,3 |
| U0126 | 1.48 ± 0.54 *1,3 |
| Sericin | 6.49 ± 0.82 *1,2 |
| Sericin + U0126 | 1.63 ± 0.68 *1,3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Fukuoka, Y.; Ishii, M.; Otake, H.; Yamamoto, T.; Taga, A.; Okamoto, N.; Shimomura, Y. Instillation of Sericin Enhances Corneal Wound Healing through the ERK Pathway in Rat Debrided Corneal Epithelium. Int. J. Mol. Sci. 2018, 19, 1123. https://doi.org/10.3390/ijms19041123
Nagai N, Fukuoka Y, Ishii M, Otake H, Yamamoto T, Taga A, Okamoto N, Shimomura Y. Instillation of Sericin Enhances Corneal Wound Healing through the ERK Pathway in Rat Debrided Corneal Epithelium. International Journal of Molecular Sciences. 2018; 19(4):1123. https://doi.org/10.3390/ijms19041123
Chicago/Turabian StyleNagai, Noriaki, Yuya Fukuoka, Miyu Ishii, Hiroko Otake, Tetsushi Yamamoto, Atsushi Taga, Norio Okamoto, and Yoshikazu Shimomura. 2018. "Instillation of Sericin Enhances Corneal Wound Healing through the ERK Pathway in Rat Debrided Corneal Epithelium" International Journal of Molecular Sciences 19, no. 4: 1123. https://doi.org/10.3390/ijms19041123