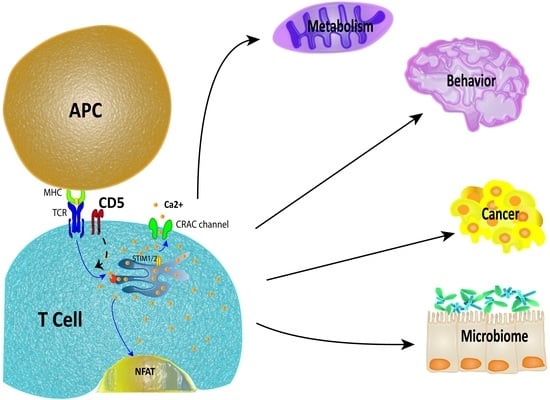
T Cell Calcium Signaling Regulation by the Co-Receptor CD5
Abstract
:1. Introduction
2. Roles of Negative Regulatory T Cell Co-Receptors
2.1. Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4)
2.2. Programmed Death 1 (PD-1)
3. CD5: A Contradictory Co-Receptor
3.1. Overview of CD5 Signaling and Ca2+ Mobilization in T Cells
3.2. CD5 as a Ca2+ Signaling Modulator
4. Physiological Impact of CD5 Expression in T Cells
4.1. Metabolism
4.2. Neuroimmunology
4.3. Cancer
4.4. Microbiome
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| CD | Cluster of differenciation |
| PD-1 | Programmed cell death protein 1 |
| AMP | Adenosine monophosphate |
| ATP | Adenosine triphosphate |
| CaMKK | Calmodulin-dependent protein kinase kinase |
| AMPK | AMP-activated protein kinase |
| SOCE | Store-operated calcium channels |
| CRAC | Calcium+-release-activated channel |
| STIM | Stromal interaction molecule |
| SERCA | Sarcoendoplasmic reticulum calcium transport ATPase |
| ER | Endoplasmic reticulum |
| NFAT | Nuclear factor of activated T cells |
| INF-γ | Interferon gamma |
| TNF | Tumor necrosis factor |
| IL-2 | Interleukin 2 |
| GLUT1 | Glucose transporter 1 |
| GLUT3 | Glucose transporter 3 |
| TIL | Tumor infiltrating lymphocytes |
| ERK | Extracellular signal-regulated kinases |
References
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, N.; Kerkau, T.; Hünig, T. CD28 co-stimulation in T cell homeostasis: A recent perspective. Immunotargets Ther. 2015, 4, 111–122. [Google Scholar] [PubMed]
- Fracchia, K.M.; Pai, C.Y.; Walsh, C.M. Modulation of T cell metabolism and function through calcium signaling. Front. Immunol. 2013, 4, 324. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.J.; Lafferty, K.J. Letter: Cellular proliferation can be an unreliable index of immune competence. J. Immunol. 1974, 112, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yamashita, M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin. Immunol. 2010, 22, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Goral, S. The three-signal hypothesis of lymphocyte activation/targets for immunosuppression. Dial. Transplant. 2011, 40, 14–16. [Google Scholar] [CrossRef]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naïve to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Abbas, A.K. T cell costimulation—Biology, therapeutic potential, and challenges. N. Engl. J. Med. 2006, 355, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Artyomov, M.N.; Lis, M.; Devadas, S.; Davis, M.M.; Chakraborty, A.K. CD4 and CD8 binding to MHC molecules primarily acts to enhance LCK delivery. Proc. Natl. Acad. Sci. USA 2010, 107, 16916–16921. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Lanier, L.L. Immune inhibitory receptors. Science 2000, 290, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480. [Google Scholar] [CrossRef] [PubMed]
- Fuertes Marraco, S.A.; Neubert, N.J.; Verdeil, G.; Speiser, D.E. Inhibitory receptors beyond T cell exhaustion. Front. Immunol. 2015, 6, 310. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Trowsdale, J. You say ITAM and I say ITIM, let’s call the whole thing off: The ambiguity of immunoreceptor signalling. Eur. J. Immunol. 2006, 36, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 costimulation: From mechanism to therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Dilek, N.; Poirier, N.; Hulin, P.; Coulon, F.; Mary, C.; Ville, S.; Vie, H.; Clémenceau, B.; Blancho, G.; Vanhove, B. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional t cells. PLoS ONE 2013, 8, e83139. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.A.; Sullivan, T.J.; Allison, J.P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 1997, 7, 885–895. [Google Scholar] [CrossRef]
- Lindsten, T.; Lee, K.P.; Harris, E.S.; Petryniak, B.; Craighead, N.; Reynolds, P.J.; Lombard, D.B.; Freeman, G.J.; Nadler, L.M.; Gray, G.S.; et al. Characterization of CTLA-4 structure and expression on human T cells. J. Immunol. 1993, 151, 3489–3499. [Google Scholar] [PubMed]
- Boise, L.H.; Minn, A.J.; Noel, P.J.; June, C.H.; Accavitti, M.A.; Lindsten, T.; Thompson, C.B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 1995, 3, 87–98. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef] [PubMed]
- Brossard, C.; Semichon, M.; Trautmann, A.; Bismuth, G. CD5 inhibits signaling at the immunological synapse without impairing its formation. J. Immunol. 2003, 170, 4623–4629. [Google Scholar] [CrossRef] [PubMed]
- Tabbekh, M.; Mokrani-Hammani, M.B.; Bismuth, G.; Mami-Chouaib, F. T cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology 2013, 2, e22841. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin. Ther. 2015, 37, 764–782. [Google Scholar] [CrossRef] [PubMed]
- De Wit, J.; Souwer, Y.; van Beelen, A.J.; de Groot, R.; Muller, F.J.; Klaasse Bos, H.; Jorritsma, T.; Kapsenberg, M.L.; de Jong, E.C.; van Ham, S.M. CD5 costimulation induces stable Th17 development by promoting IL-23R expression and sustained STAT3 activation. Blood 2011, 118, 6107–6114. [Google Scholar] [CrossRef] [PubMed]
- Perez-Villar, J.J.; Whitney, G.S.; Bowen, M.A.; Hewgill, D.H.; Aruffo, A.A.; Kanner, S.B. CD5 negatively regulates the T cell antigen receptor signal transduction pathway: Involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol. Cell. Biol. 1999, 19, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Gary-Gouy, H.; Harriague, J.; Dalloul, A.; Donnadieu, E.; Bismuth, G. CD5-negative regulation of B cell receptor signaling pathways originates from tyrosine residue Y429 outside an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 2002, 168, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, K.M.; Broszeit, R.; Garnett, D.; Durrheim, G.A.; Spruyt, L.L.; Beyers, A.D. Thymocyte activation induces the association of phosphatidylinositol 3-kinase and pp120 with CD5. Eur. J. Immunol. 1997, 27, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Samelson, L.E. Signal transduction mediated by the T cell antigen receptor: The role of adapter proteins. Annu. Rev. Immunol. 2002, 20, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Burgess, K.E.; Yamamoto, M.; Prasad, K.V.S.; Rudd, C.E. CD5 acts as a tyrosine kinase substrate within a receptor complex comprising T cell receptor ζ-chain CD3 and protein-tyrosine kinases P56LCK and P59FYN. Proc. Natl. Acad. Sci. USA 1992, 89, 9311–9315. [Google Scholar] [CrossRef] [PubMed]
- Consuegra-Fernandez, M.; Aranda, F.; Simoes, I.; Orta, M.; Sarukhan, A.; Lozano, F. CD5 as a Target for Immune-Based Therapies. Crit. Rev. Immunol. 2015, 35, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Roa, N.S.; Ordonez-Rueda, D.; Chavez-Rios, J.R.; Raman, C.; Garcia-Zepeda, E.A.; Lozano, F.; Soldevila, G. The carboxy-terminal region of CD5 is required for c-CBL mediated TCR signaling downmodulation in thymocytes. Biochem. Biophys. Res. Commun. 2013, 432, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Berney, S.M.; Schaan, T.; Wolf, R.E.; Kimpel, D.L.; van der Heyde, H.; Atkinson, T.P. CD5 (OKT1) augments CD3-mediated intracellular signaling events in human T lymphocytes. Inflammation 2001, 25, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Azzam, H.S.; DeJarnette, J.B.; Huang, K.; Emmons, R.; Park, C.-S.; Sommers, C.L.; El-Khoury, D.; Shores, E.W.; Love, P.E. Fine tuning of TCR signaling by CD5. J. Immunol. 2001, 166, 5464–5472. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.M.T.; Hamblin, G.J.; Raymond, C.M.; Weber, K.S. Naive helper T cells with high CD5 expression have increased calcium signaling. PLoS ONE 2017, 12, e0178799. [Google Scholar] [CrossRef] [PubMed]
- Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Reicher, B.; Barda-Saad, M. The calcium feedback loop and T cell activation: How cytoskeleton networks control intracellular calcium flux. Biochim. Biophys. Acta Biomembr. 2014, 1838, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; Kinet, J.-P. Calcium signaling in immune cells. Nat. Immunol. 2009, 10, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.M.A.; Guse, A.H. Ca2+ microdomains in T-lymphocytes. Front. Oncol. 2017, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Lewis, R.S.; Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010, 28, 491–533. [Google Scholar] [CrossRef] [PubMed]
- Oh-hora, M.; Rao, A. Calcium signaling in lymphocytes. Curr. Opin. Immunol. 2008, 20, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr. The co-receptor function of CD4. Semin. Immunol. 1991, 3, 153–160. [Google Scholar] [PubMed]
- Moran, A.E.; Hogquist, K.A. T cell receptor affinity in thymic development. Immunology 2012, 135, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Kyttaris, V.C.; Zhang, Z.; Kampagianni, O.; Tsokos, G.C. Calcium signaling in systemic lupus erythematosus T cells: A treatment target. Arthritis Rheum. 2011, 63, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U.; Winklewski, P.; Ciepiela, O.; Popko, K.; Lipinska, A.; Kucharska, A.; Michalska, B.; Wasik, M. Modulatory effect of insulin on T cell receptor mediated calcium signaling is blunted in long lasting type 1 diabetes mellitus. Pharmacol. Rep. 2012, 64, 150–156. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T cell activation by distinct mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Grosso, J.F.; Jure-Kunkel, M.N. CTLA-4 blockade in tumor models: An overview of preclinical and translational research. Cancer Immun. 2013, 13, 5. [Google Scholar] [PubMed]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Jago, C.B.; Yates, J.; Olsen Saraiva CÂMara, N.; Lechler, R.I.; Lombardi, G. Differential expression of CTLA-4 among T cell subsets. Clin. Exp. Immunol. 2004, 136, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Bradshaw, J.; Greene, J.; Peach, R.; Bennett, K.L.; Mittler, R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996, 4, 535–543. [Google Scholar] [CrossRef]
- Schneider, H.; Smith, X.; Liu, H.; Bismuth, G.; Rudd, C.E. CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur. J. Immunol. 2008, 38, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Orabona, C.; Fallarino, F.; Vacca, C.; Calcinaro, F.; Falorni, A.; Candeloro, P.; Belladonna, M.L.; Bianchi, R.; Fioretti, M.C.; et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002, 3, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Wahl, S.M. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-β) production by murine CD4+ T cells. J. Exp. Med. 1998, 188, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, A.; Boasso, A.; Edghill-Smith, Y.; Vaccari, M.; Fuchs, D.; Venzon, D.; Nacsa, J.; Betts, M.R.; Tsai, W.-P.; Heraud, J.-M.; et al. CTLA-4 blockade decreases TGF-β, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 2006, 108, 3834–3842. [Google Scholar] [CrossRef] [PubMed]
- Iken, K.; Liu, K.; Liu, H.; Bizargity, P.; Wang, L.; Hancock, W.W.; Visner, G.A. Indoleamine 2,3-dioxygenase and metabolites protect murine lung allografts and impair the calcium mobilization of T cells. Am. J. Respir. Cell Mol. Biol. 2012, 47, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.K.; Sansom, D.M. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol. 2015, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cederbom, L.; Hall, H.; Ivars, F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 2000, 30, 1538–1543. [Google Scholar] [CrossRef]
- Burnett, D.L.; Parish, I.A.; Masle-Farquhar, E.; Brink, R.; Goodnow, C.C. Murine LRBA deficiency causes CTLA-4 deficiency in Tregs without progression to immune dysregulation. Immunol. Cell Biol. 2017, 95, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Burns, S.O.; Walker, L.S.K.; Sansom, D.M. Immune deficiency and autoimmunity in patients with CTLA-4 (CD152) mutations. Clin. Exp. Immunol. 2017, 190, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Hughson, A.; Fowell, D.J. CTLA-4 is Required by CD4+CD25+ treg to control CD4+ T cell lymphopenia-induced proliferation. Eur. J. Immunol. 2009, 39, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, O.P.; Larsen, Z.M.; Pociot, F. CTLA-4 in autoimmune diseases--a general susceptibility gene to autoimmunity? Genes Immun. 2000, 1, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Chikuma, S. CTLA-4, an essential immune-checkpoint for T cell activation. Curr. Top. Microbiol. Immunol. 2017, 410, 99–126. [Google Scholar] [PubMed]
- Tai, X.; Van Laethem, F.; Pobezinsky, L.; Guinter, T.; Sharrow, S.O.; Adams, A.; Granger, L.; Kruhlak, M.; Lindsten, T.; Thompson, C.B.; et al. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood 2012, 119, 5155–5163. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Abdel-Motal, U.M. Lessons from CTLA-4 deficiency and checkpoint inhibition. Curr. Opin. Immunol. 2017, 49, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Yuan, J.; Yang, A.; Schaer, D.; Wolchok, J.D. Modulation of CTLA-4 and GITR for cancer immunotherapy. Curr. Top. Microbiol. Immunol. 2011, 344, 211–244. [Google Scholar] [PubMed]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A., Jr.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.Y.; Gore, I.; Fong, L.; Venook, A.; Beck, S.B.; Dorazio, P.; Criscitiello, P.J.; Healey, D.I.; Huang, B.; Gomez-Navarro, J.; et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 3485–3490. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Camacho, L.H.; Lopez-Berestein, G.; Pavlov, D.; Bulanhagui, C.A.; Millham, R.; Comin-Anduix, B.; Reuben, J.M.; Seja, E.; Parker, C.A.; et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 2005, 23, 8968–8977. [Google Scholar] [CrossRef] [PubMed]
- Calabro, L.; Morra, A.; Fonsatti, E.; Cutaia, O.; Fazio, C.; Annesi, D.; Lenoci, M.; Amato, G.; Danielli, R.; Altomonte, M.; et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: An open-label, single-arm, phase 2 study. Lancet Respir. Med. 2015, 3, 301–309. [Google Scholar] [CrossRef]
- Comin-Anduix, B.; Escuin-Ordinas, H.; Ibarrondo, F.J. Tremelimumab: Research and clinical development. OncoTargets Ther. 2016, 9, 1767–1776. [Google Scholar]
- Ribas, A.; Comin-Anduix, B.; Chmielowski, B.; Jalil, J.; de la Rocha, P.; McCannel, T.A.; Ochoa, M.T.; Seja, E.; Villanueva, A.; Oseguera, D.K.; et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin. Cancer Res. 2009, 15, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.; Goldberg, S.B.; Balmanoukian, A.; Chaft, J.E.; Sanborn, R.E.; Gupta, A.; Narwal, R.; Steele, K.; Gu, Y.; Karakunnel, J.J.; et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol. 2016, 17, 299–308. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.; Yang, L.; Li, Y. The role of PD-1 and PD-L1 in T cell immune suppression in patients with hematological malignancies. J. Hematol. Oncol. 2013, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Wang, J. PD-1/PD-L pathway and autoimmunity. Autoimmunity 2005, 38, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Boussiotis, V.A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-F.; Fouquet, S.; Chapon, M.; Salmon, H.; Regnier, F.; Labroquère, K.; Badoual, C.; Damotte, D.; Validire, P.; Maubec, E.; et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS ONE 2011, 6, e17621. [Google Scholar] [CrossRef] [PubMed]
- Gorentla, B.K.; Zhong, X.-P. T cell receptor signal transduction in T lymphocytes. J. Clin. Cell. Immunol. 2012, 2012, 005. [Google Scholar]
- Wei, F.; Zhong, S.; Ma, Z.; Kong, H.; Medvec, A.; Ahmed, R.; Freeman, G.J.; Krogsgaard, M.; Riley, J.L. Strength of PD-1 signaling differentially affects T cell effector functions. Proc. Natl. Acad. Sci. USA 2013, 110, E2480–E2489. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Chaudhari, S.M.; Koch, M.; Wiendl, H.; Eckstein, H.-H.; Zernecke, A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS ONE 2014, 9, e93280. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Kishi, Y.; Meguri, Y.; Yoshioka, T.; Iwamoto, M.; Maeda, Y.; Yagita, H.; Tanimoto, M.; Koreth, J.; Ritz, J.; et al. PD-1 signaling has a critical role in maintaining regulatory T cell homeostasis; implication for treg depletion therapy by PD-1 blockade. Blood 2015, 126, 848. [Google Scholar]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, M.; Zhang, B.; Sugiura, Y.; Sonomura, K.; Guerrini, M.M.; Tsutsui, Y.; Maruya, M.; Vogelzang, A.; Chamoto, K.; Honda, K.; et al. Metabolic shift induced by systemic activation of T cells in PD-1-deficient mice perturbs brain monoamines and emotional behavior. Nat. Immunol. 2017, 18, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- Kroner, A.; Mehling, M.; Hemmer, B.; Rieckmann, P.; Toyka, K.V.; Maurer, M.; Wiendl, H. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann. Neurol 2005, 58, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Adamska, E.; Nowak, O.; Karabon, L.; Pokryszko-Dragan, A.; Partyka, A.; Tomkiewicz, A.; Ptaszkowski, J.; Frydecka, I.; Podemski, R.; Dybko, J.; et al. PD-1 gene polymorphic variation is linked with first symptom of disease and severity of relapsing-remitting form of MS. J. Neuroimmunol. 2017, 305, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Jia, R.; Zhang, X.; Fang, Q.; Huang, L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell. Immunol. 2014, 290, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun. Rev. 2013, 12, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yoshida, T.; Nakaki, F.; Hiai, H.; Okazaki, T.; Honjo, T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. USA 2005, 102, 11823–11828. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Otaka, Y.; Wang, J.; Hiai, H.; Takai, T.; Ravetch, J.V.; Honjo, T. Hydronephrosis associated with antiurothelial and antinuclear autoantibodies in BALB/c-Fcgr2b−/−Pdcd1−/− mice. J. Exp. Med. 2005, 202, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, S.; Zhu, B.; Bedoret, D.; Bu, X.; Francisco, L.M.; Hua, P.; Duke-Cohan, J.S.; Umetsu, D.T.; Sharpe, A.H.; et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J. Exp. Med. 2014, 211, 943–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, K.; Kishimoto, T. CD5: A new partner for IL-6. Immunity 2016, 44, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Jones, N.H.; Strominger, J.L.; Herzenberg, L.A. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: Molecular homology to its human counterpart Leu-1/T1 (CD5). Proc. Natl. Acad. Sci. USA 1987, 84, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Tarakhovsky, A.; Kanner, S.B.; Hombach, J.; Ledbetter, J.A.; Muller, W.; Killeen, N.; Rajewsky, K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 1995, 269, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, A. CD5: A safeguard against autoimmunity and a shield for cancer cells. Autoimmun. Rev. 2009, 8, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Bhandoola, A.; Bosselut, R.; Yu, Q.; Cowan, M.L.; Feigenbaum, L.; Love, P.E.; Singer, A. CD5-mediated inhibition of TCR signaling during intrathymic selection and development does not require the CD5 extracellular domain. Eur. J. Immunol. 2002, 32, 1811–1817. [Google Scholar] [CrossRef]
- Mandl, J.N.; Monteiro, J.P.; Vrisekoop, N.; Germain, R.N. T cell positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 2013, 38, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.G.; Opejin, A.; Jones, A.; Gross, C.; Hawiger, D. CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity 2015, 42, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; de Leij, L.F.; Wayman, G.A.; Tokumitsu, H.; Vellenga, E. The Ca2+/calmodulin-dependent kinase type IV is involved in the CD5-mediated signaling pathway in human T lymphocytes. J. Biol. Chem. 1997, 272, 31809–31820. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.J.; Simmonds, S.J.; Clarkson, N.G.; Hanrahan, S.; Puklavec, M.J.; Bomb, M.; Barclay, A.N.; Brown, M.H. CD6 regulates T cell responses through activation-dependent recruitment of the positive regulator SLP-76. Mol. Cell. Biol. 2006, 26, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Pena-Rossi, C.; Zuckerman, L.A.; Strong, J.; Kwan, J.; Ferris, W.; Chan, S.; Tarakhovsky, A.; Beyers, A.D.; Killeen, N. Negative regulation of CD4 lineage development and responses by CD5. J. Immunol. 1999, 163, 6494–6501. [Google Scholar] [PubMed]
- Davies, A.A.; Ley, S.C.; Crumpton, M.J. CD5 is phosphorylated on tyrosine after stimulation of the T cell antigen receptor complex. Proc. Natl. Acad. Sci. USA 1992, 89, 6368–6372. [Google Scholar] [CrossRef] [PubMed]
- Samelson, L.E.; Phillips, A.F.; Luong, E.T.; Klausner, R.D. Association of the fyn protein-tyrosine kinase with the T cell antigen receptor. Proc. Natl. Acad. Sci. USA 1990, 87, 4358–4362. [Google Scholar] [CrossRef] [PubMed]
- Raab, M.; Yamamoto, M.; Rudd, C.E. The T cell antigen CD5 acts as a receptor and substrate for the protein-tyrosine kinase p56lck. Mol.Cell. Biol. 1994, 14, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Beyers, A.D.; Spruyt, L.L.; Williams, A.F. Molecular associations between the T-lymphocyte antigen receptor complex and the surface antigens CD2, CD4, or CD8 and CD5. Proc. Natl. Acad. Sci. USA 1992, 89, 2945–2949. [Google Scholar] [CrossRef] [PubMed]
- Spertini, F.; Stohl, W.; Ramesh, N.; Moody, C.; Geha, R.S. Induction of human T cell proliferation by a monoclonal antibody to CD5. J. Immunol. 1991, 146, 47–52. [Google Scholar] [PubMed]
- Persaud, S.P.; Parker, C.R.; Lo, W.-L.; Weber, K.S.; Allen, P.M. Intrinsic CD4+ T cell sensitivity and response to pathogen are set and sustained by avidity for thymic and peripheral self-pMHC. Nat. Immunol. 2014, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Padilla, O.; Places, L.; Vigorito, E.; Vila, J.M.; Vilella, R.; Mila, J.; Vives, J.; Bowen, M.A.; Lozano, F. Relevance of individual CD5 extracellular domains on antibody recognition, glycosylation and co-mitogenic signalling. Tissue Antigen. 1999, 54, 16–26. [Google Scholar] [CrossRef]
- McAlister, M.S.; Brown, M.H.; Willis, A.C.; Rudd, P.M.; Harvey, D.J.; Aplin, R.; Shotton, D.M.; Dwek, R.A.; Barclay, A.N.; Driscoll, P.C. Structural analysis of the CD5 antigen—Expression, disulphide bond analysis and physical characterisation of CD5 scavenger receptor superfamily domain 1. Eur J. Biochem. 1998, 257, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Kim, H.-O.; Surh, C.D.; Sprent, J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity 2010, 32, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Yashiro-Ohtani, Y.; Zhou, X.-Y.; Toyo-oka, K.; Tai, X.-G.; Park, C.-S.; Hamaoka, T.; Abe, R.; Miyake, K.; Fujiwara, H. Non-CD28 costimulatory molecules present in T cell rafts induce T cell costimulation by enhancing the association of TCR with rafts. J. Immunol. 2000, 164, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- König, R. Chapter 315—Signal Transduction in T Lymphocytes A2—Bradshaw, Ralph A. In Handbook of Cell Signaling, 2nd ed.; Dennis, E.A., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 2679–2688. [Google Scholar]
- Milam, A.V.; Allen, P.M. Functional heterogeneity in CD4+ T cell responses against a bacterial pathogen. Front. Immunol 2015, 6, 621. [Google Scholar] [CrossRef] [PubMed]
- Lozano, F.; Simarro, M.; Calvo, J.; Vila, J.M.; Padilla, O.; Bowen, M.A.; Campbell, K.S. CD5 signal transduction: Positive or negative modulation of antigen receptor signaling. Crit. Rev. Immunol. 2000, 20, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Hogquist, K.A.; Jameson, S.C. The self-obsession of T cells: How TCR signaling thresholds affect fate decisions in the thymus and effector function in the periphery. Nat. Immunol. 2014, 15, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, H.; von Hoegen, I.; Luo, W.; Parnes, J.R.; Thielemans, K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature 1991, 351, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Biancone, L.; Bowen, M.A.; Lim, A.; Aruffo, A.; Andres, G.; Stamenkovic, I. Identification of a novel inducible cell-surface ligand of CD5 on activated lymphocytes. J. Exp. Med. 1996, 184, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Lacey, E. A ligand for CD5 is CD5. J. Immunol. 2010, 185, 6068–6074. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Van de Velde, H.; von Hoegen, I.; Parnes, J.R.; Thielemans, K. Ly-1 (CD5), a membrane glycoprotein of mouse T lymphocytes and a subset of B cells, is a natural ligand of the B cell surface protein Lyb-2 (CD72). J. Immunol. 1992, 148, 1630–1634. [Google Scholar] [PubMed]
- Vandenberghe, P.; Verwilghen, J.; Van Vaeck, F.; Ceuppens, J.L. Ligation of the CD5 or CD28 molecules on resting human T cells induces expression of the early activation antigen CD69 by a calcium- and tyrosine kinase-dependent mechanism. Immunology 1993, 78, 210–217. [Google Scholar] [PubMed]
- Ceuppens, J.L.; Baroja, M.L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J. Immunol. 1986, 137, 1816–1821. [Google Scholar] [PubMed]
- June, C.H.; Rabinovitch, P.S.; Ledbetter, J.A. CD5 antibodies increase intracellular ionized calcium concentration in T cells. J. Immunol. 1987, 138, 2782–2792. [Google Scholar] [PubMed]
- Reth, M. Antigen receptor tail clue. Nature 1989, 338, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Unkeless, J.C.; Jin, J. Inhibitory receptors, ITIM sequences and phosphatases. Curr. Opin. Immunol 1997, 9, 338–343. [Google Scholar] [CrossRef]
- Dong, B.; Somani, A.K.; Love, P.E.; Zheng, X.; Chen, X.; Zhang, J. CD5-mediated inhibition of TCR signaling proceeds normally in the absence of SHP-1. Int. J. Mol. Med. 2016, 38, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.S.; Li, Q.J.; Persaud, S.P.; Campbell, J.D.; Davis, M.M.; Allen, P.M. Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proc. Natl. Acad. Sci. USA 2012, 109, 9511–9516. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.B.; Hamilton, S.E.; Xing, Y.; Best, J.A.; Goldrath, A.W.; Hogquist, K.A.; Jameson, S.C. The TCR’s sensitivity to self peptide–MHC dictates the ability of naive CD8+ T cells to respond to foreign antigens. Nat. Immunol. 2014, 16, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Palin, A.C.; Love, P.E. CD5 helps aspiring regulatory T cells ward off unwelcome cytokine advances. Immunity 2015, 42, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Chan, S.L. Calcium orchestrates apoptosis. Nat. Cell Biol. 2003, 5, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Nicotera, P. The calcium ion and cell death. J. Neural Transm. Suppl. 1994, 43, 1–11. [Google Scholar] [PubMed]
- Zhao, C.; Davies, J.D. A peripheral CD4+ T cell precursor for naive, memory, and regulatory T cells. J. Exp. Med. 2010, 207, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.R.; Byersdorfer, C.A.; Ferrara, J.L.M.; Opipari, A.W.; Glick, G.D. Distinct metabolic programs in activated T cells: Opportunities for selective immunomodulation. Immunol. Rev. 2012, 249, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Van der Windt, G.J.; Pearce, E.L. Metabolic switching and fuel choice during T cell differentiation and memory development. Immunol. Rev. 2012, 249, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Curtis, J.D.; Maggi, L.B., Jr.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.-C.; van der Windt, G.J.W.; Blagih, J.; Qiu, J.; et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Lochner, M.; Berod, L.; Sparwasser, T. Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016, 28, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Vaeth, M.; Maus, M.; Klein-Hessling, S.; Freinkman, E.; Yang, J.; Eckstein, M.; Cameron, S.; Turvey, S.E.; Serfling, E.; Berberich-Siebelt, F.; et al. Store-operated Ca2+ entry controls clonal expansion of T cells through metabolic reprogramming. Immunity 2017, 47, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Tamás, P.; Hawley, S.A.; Clarke, R.G.; Mustard, K.J.; Green, K.; Hardie, D.G.; Cantrell, D.A. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 2006, 203, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.H.; Poffenberger, M.C.; Wong, A.H.; Jones, R.G. The role of AMPK in T cell metabolism and function. Curr. Opin. Immunol. 2017, 46, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Manning, B.D. The TSC1–TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Regulation and function of mTOR signalling in T cell fate decision. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- MacIver, N.J.; Blagih, J.; Saucillo, D.C.; Tonelli, L.; Griss, T.; Rathmell, J.C.; Jones, R.G. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol. 2011, 187, 4187–4198. [Google Scholar] [CrossRef] [PubMed]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Gilabert, J.A.; Bakowski, D.; Parekh, A.B. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J. 2001, 20, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Gilabert, J.A.; Parekh, A.B. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current I (CRAC). EMBO J. 2000, 19, 6401–6407. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, K.; Nelson, C.; Bakowski, D.; de Brito, O.M.; Ng, S.W.; di Capite, J.; Powell, T.; Scorrano, L.; Parekh, A.B. Mitofusin 2 regulates STIM1 migration from the Ca2+ store to the plasma membrane in cells with depolarized mitochondria. J. Biol. Chem. 2011, 286, 12189–12201. [Google Scholar] [CrossRef] [PubMed]
- Dimeloe, S.; Burgener, A.V.; Grahlert, J.; Hess, C. T cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology 2017, 150, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jouaville, L.S.; Pinton, P.; Bastianutto, C.; Rutter, G.A.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 1999, 96, 13807–13812. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Rumi-Masante, J.; Rusinga, F.I.; Lester, T.E.; Dunlap, T.B.; Williams, T.D.; Dunker, A.K.; Weis, D.D.; Creamer, T.P. Structural basis for activation of calcineurin by calmodulin. J. Mol. Biol. 2012, 415, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Racioppi, L.; Means, A.R. Calcium/calmodulin-dependent protein kinase kinase 2: Roles in signaling and pathophysiology. J. Biol. Chem. 2012, 287, 31658–31665. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 alters T cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef] [PubMed]
- Gary-Gouy, H.; Sainz-Perez, A.; Marteau, J.-B.; Marfaing-Koka, A.; Delic, J.; Merle-Beral, H.; Galanaud, P.; Dalloul, A. Natural phosphorylation of CD5 in chronic lymphocytic leukemia B cells and analysis of CD5-regulated genes in a B cell line suggest a role for CD5 in malignant phenotype. J. Immunol. 2007, 179, 4335–4344. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.J.; Mahajan, V.S.; Chen, J.; Irvine, D.J.; Lauffenburger, D.A. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8+ T cells. Immunol Cell. Biol. 2011, 89, 581–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipnis, J.; Gadani, S.; Derecki, N.C. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 2012, 12, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, J.; Cohen, H.; Cardon, M.; Ziv, Y.; Schwartz, M. T cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 8180–8185. [Google Scholar] [CrossRef] [PubMed]
- Brombacher, T.M.; Nono, J.K.; De Gouveia, K.S.; Makena, N.; Darby, M.; Womersley, J.; Tamgue, O.; Brombacher, F. IL-13–mediated regulation of learning and memory. J. Immunol. 2017, 198, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-dos-Santos, A.J.; Matsumoto, G.; Snow, B.E.; Bai, D.; Houston, F.P.; Whishaw, I.Q.; Mariathasan, S.; Sasaki, T.; Wakeham, A.; Ohashi, P.S.; et al. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc. Natl. Acad. Sci. USA 2000, 97, 12272–12277. [Google Scholar] [CrossRef] [PubMed]
- Filiano, A.J.; Gadani, S.P.; Kipnis, J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci. 2017, 18, 375. [Google Scholar] [CrossRef] [PubMed]
- Kyratsous, N.I.; Bauer, I.J.; Zhang, G.; Pesic, M.; Bartholomäus, I.; Mues, M.; Fang, P.; Wörner, M.; Everts, S.; Ellwart, J.W.; et al. Visualizing context-dependent calcium signaling in encephalitogenic T cells in vivo by two-photon microscopy. Proc. Natl. Acad. Sci. USA 2017, 114, E6381–E6389. [Google Scholar] [CrossRef] [PubMed]
- Smedler, E.; Uhlén, P. Frequency decoding of calcium oscillations. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Pesic, M.; Bartholomaus, I.; Kyratsous, N.I.; Heissmeyer, V.; Wekerle, H.; Kawakami, N. 2-photon imaging of phagocyte-mediated T cell activation in the CNS. J. Clin. Investig. 2013, 123, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, N.M.W.J.; Schmitz, K.; Schiffmann, S.; Tafferner, N.; Schmidt, M.; Jordan, H.; Häußler, A.; Tegeder, I.; Geisslinger, G.; Parnham, M.J. Multiple rodent models and behavioral measures reveal unexpected responses to FTY720 and DMF in experimental autoimmune encephalomyelitis. Behav. Brain Res. 2016, 300, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Schub, D.; Janssen, E.; Leyking, S.; Sester, U.; Assmann, G.; Hennes, P.; Smola, S.; Vogt, T.; Rohrer, T.; Sester, M.; et al. Altered phenotype and functionality of varicella zoster virus–specific cellular immunity in individuals with active infection. J. Infect. Dis. 2015, 211, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Schub, D.; Fousse, M.; Faßbender, K.; Gärtner, B.C.; Sester, U.; Sester, M.; Schmidt, T. CTLA-4-expression on VZV-specific T cells in CSF and blood is specifically increased in patients with VZV related central nervous system infections. Eur. J. Immunol. 2018, 48, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Koebel, C.M.; Vermi, W.; Swann, J.B.; Zerafa, N.; Rodig, S.J.; Old, L.J.; Smyth, M.J.; Schreiber, R.D. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007, 450, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Mattes, J.; Hulett, M.; Xie, W.; Hogan, S.; Rothenberg, M.E.; Foster, P.; Parish, C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J. Exp. Med. 2003, 197, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Hayashi, R.; Lafond-Walker, A.; Lowenstein, C.; Pardoll, D.; Levitsky, H. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 1998, 188, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Scholler, J.; Brady, T.L.; Binder-Scholl, G.; Hwang, W.T.; Plesa, G.; Hege, K.M.; Vogel, A.N.; Kalos, M.; Riley, J.L.; Deeks, S.G.; et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012, 4, 132ra153. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.S.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Huetter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Muessig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kuecherer, C.; Blau, O.; et al. Long-Term Control of HIV by CCR5 Δ32/Δ32 Stem-Cell Transplantaion. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Baitsch, L.; Baumgaertner, P.; Devevre, E.; Raghav, S.K.; Legat, A.; Barba, L.; Wieckowski, S.; Bouzourene, H.; Deplancke, B.; Romero, P.; et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Investig. 2011, 121, 2350–2360. [Google Scholar] [CrossRef] [PubMed]
- Staveley-O’Carroll, K.; Sotomayor, E.; Montgomery, J.; Borrello, I.; Hwang, L.; Fein, S.; Pardoll, D.; Levitsky, H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc. Natl. Acad. Sci. USA 1998, 95, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Wunderlich, J.R.; Robbins, P.F.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.; Restifo, N.P.; Hubicki, A.M.; et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002, 298, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.-A.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’er, D.; et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 2017, 170, 1120–1133.e17. [Google Scholar] [CrossRef] [PubMed]
- Barbee, M.S.; Ogunniyi, A.; Horvat, T.Z.; Dang, T.O. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann. Pharmacother. 2015, 49, 907–937. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Inarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P.; Gowrishankar, K. Pembrolizumab joins the anti-PD-1 armamentarium in the treatment of melanoma. Future Oncol. 2015, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Rooke, R. Can calcium signaling be harnessed for cancer immunotherapy? Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Lee, D.S.; Chang, J.M.; Sprent, J.; Surh, C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 1999, 11, 173–181. [Google Scholar] [CrossRef]
- Smith, K.; Seddon, B.; Purbhoo, M.A.; Zamoyska, R.; Fisher, A.G.; Merkenschlager, M. Sensory adaptation in naive peripheral CD4 T cells. J. Exp. Med. 2001, 194, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Dorothée, G.; Vergnon, I.; El Hage, F.; Chansac, B.L.M.; Ferrand, V.; Lécluse, Y.; Opolon, P.; Chouaib, S.; Bismuth, G.; Mami-Chouaib, F. In situ sensory adaptation of tumor-infiltrating T lymphocytes to peptide-MHC levels elicits strong antitumor reactivity. J. Immunol. 2005, 174, 6888–6897. [Google Scholar] [CrossRef] [PubMed]
- Friedlein, G.; El Hage, F.; Vergnon, I.; Richon, C.; Saulnier, P.; Lecluse, Y.; Caignard, A.; Boumsell, L.; Bismuth, G.; Chouaib, S.; et al. Human CD5 protects circulating tumor antigen-specific CTL from tumor-mediated activation-induced cell death. J. Immunol. 2007, 178, 6821–6827. [Google Scholar] [CrossRef] [PubMed]
- Axtell, R.C.; Webb, M.S.; Barnum, S.R.; Raman, C. Cutting edge: Critical role for CD5 in experimental autoimmune encephalomyelitis: Inhibition of engagement reverses disease in mice. J. Immunol. 2004, 173, 2928–2932. [Google Scholar] [CrossRef] [PubMed]
- Simoes, I.T.; Aranda, F.; Carreras, E.; Andres, M.V.; Casado-Llombart, S.; Martinez, V.G.; Lozano, F. Immunomodulatory effects of soluble CD5 on experimental tumor models. Oncotarget 2017, 8, 108156–108169. [Google Scholar] [CrossRef] [PubMed]
- Tabbekh, M.; Franciszkiewicz, K.; Haouas, H.; Lecluse, Y.; Benihoud, K.; Raman, C.; Mami-Chouaib, F. Rescue of tumor-infiltrating lymphocytes from activation-induced cell death enhances the antitumor CTL response in CD5-deficient mice. J. Immunol. 2011, 187, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiome shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Zizzari, I.; Mazzuca, F.; Ascierto, P.A.; Putignani, L.; Marchetti, L.; Napoletano, C.; Nuti, M.; Marchetti, P. Cross-talk between microbiota and immune fitness to steer and control response to anti PD-1/PDL-1 treatment. Oncotarget 2017, 8, 8890–8899. [Google Scholar] [CrossRef] [PubMed]
- Kosiewicz, M.M.; Dryden, G.W.; Chhabra, A.; Alard, P. Relationship between gut microbiota and development of T cell associated disease. FEBS Lett. 2014, 588, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.-W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.-S. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, G.; Lamousé-Smith, E.S.N. Gastrointestinal microbiome dysbiosis in infant mice alters peripheral CD8+ T cell receptor signaling. Front. Immunol. 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wei, B.; Velazquez, P.; Borneman, J.; Braun, J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naïve CD4+ T lymphocytes. Clin. Immunol. 2005, 117, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bazett, M.; Bergeron, M.-E.; Haston, C.K. Streptomycin treatment alters the intestinal microbiome, pulmonary T cell profile and airway hyperresponsiveness in a cystic fibrosis mouse model. Sci. Rep. 2016, 6, 19189. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Haque-Begum, S.; Kasper, L.H. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes 2010, 1, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.P.; Krummel, M.F. The Yin and Yang of T cell costimulation. Science 1995, 270, 932–933. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.P. Checkpoints. Cell 2015, 162, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, C.M.T.; Johnson, D.K.; Weber, K.S. T Cell Calcium Signaling Regulation by the Co-Receptor CD5. Int. J. Mol. Sci. 2018, 19, 1295. https://doi.org/10.3390/ijms19051295
Freitas CMT, Johnson DK, Weber KS. T Cell Calcium Signaling Regulation by the Co-Receptor CD5. International Journal of Molecular Sciences. 2018; 19(5):1295. https://doi.org/10.3390/ijms19051295
Chicago/Turabian StyleFreitas, Claudia M. Tellez, Deborah K. Johnson, and K. Scott Weber. 2018. "T Cell Calcium Signaling Regulation by the Co-Receptor CD5" International Journal of Molecular Sciences 19, no. 5: 1295. https://doi.org/10.3390/ijms19051295
APA StyleFreitas, C. M. T., Johnson, D. K., & Weber, K. S. (2018). T Cell Calcium Signaling Regulation by the Co-Receptor CD5. International Journal of Molecular Sciences, 19(5), 1295. https://doi.org/10.3390/ijms19051295