Knockout of Pannexin-1 Induces Hearing Loss
Abstract
:1. Introduction
2. Results
2.1. Panx1 Deletion in the Cochlea in Gen-Panx1 KO Mice
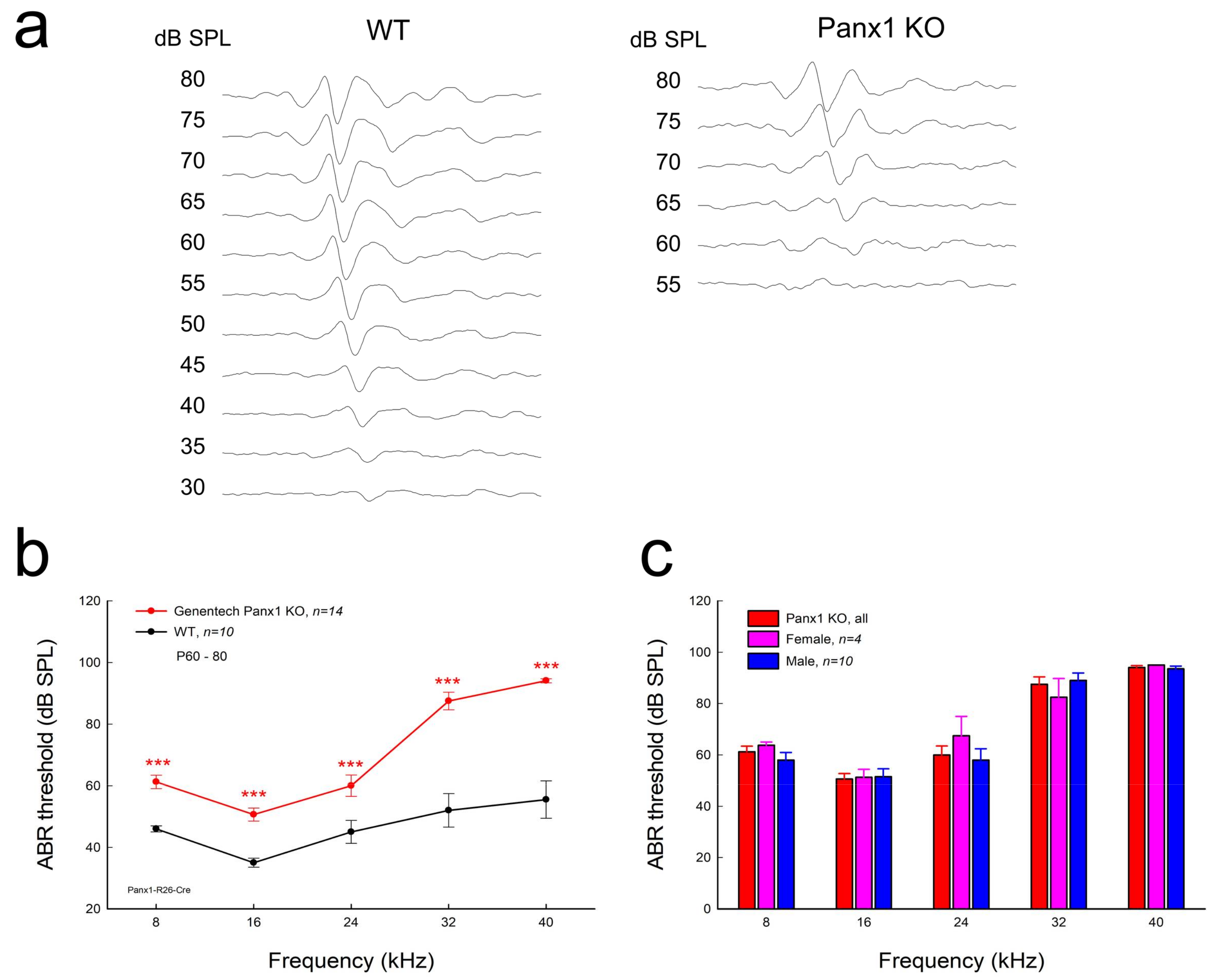
2.2. Hearing Loss in Gen-Panx1 KO Mice
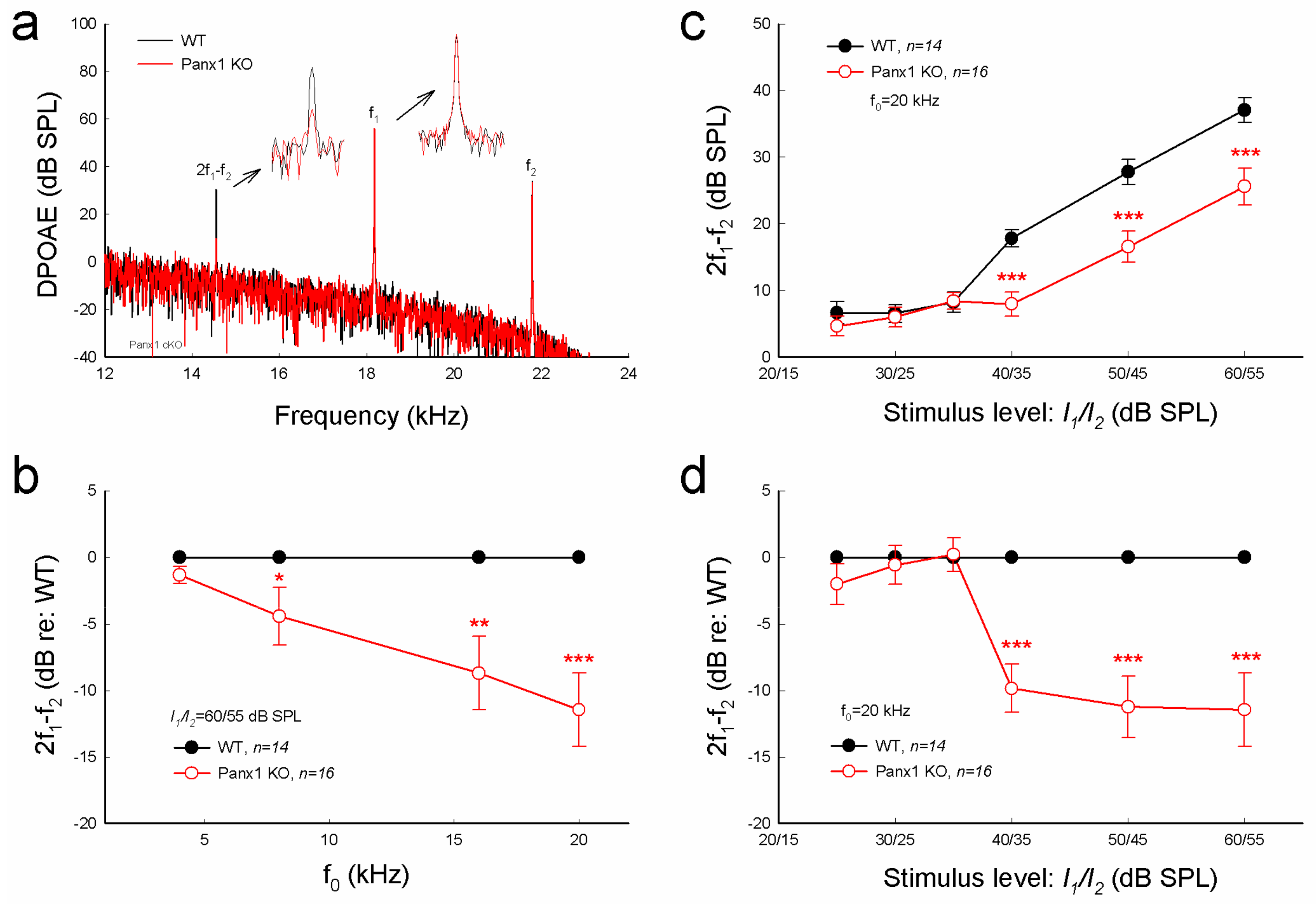
2.3. Reduction of Distortion Product Otoacoustic Emission in Gen-Panx1 KO Mice
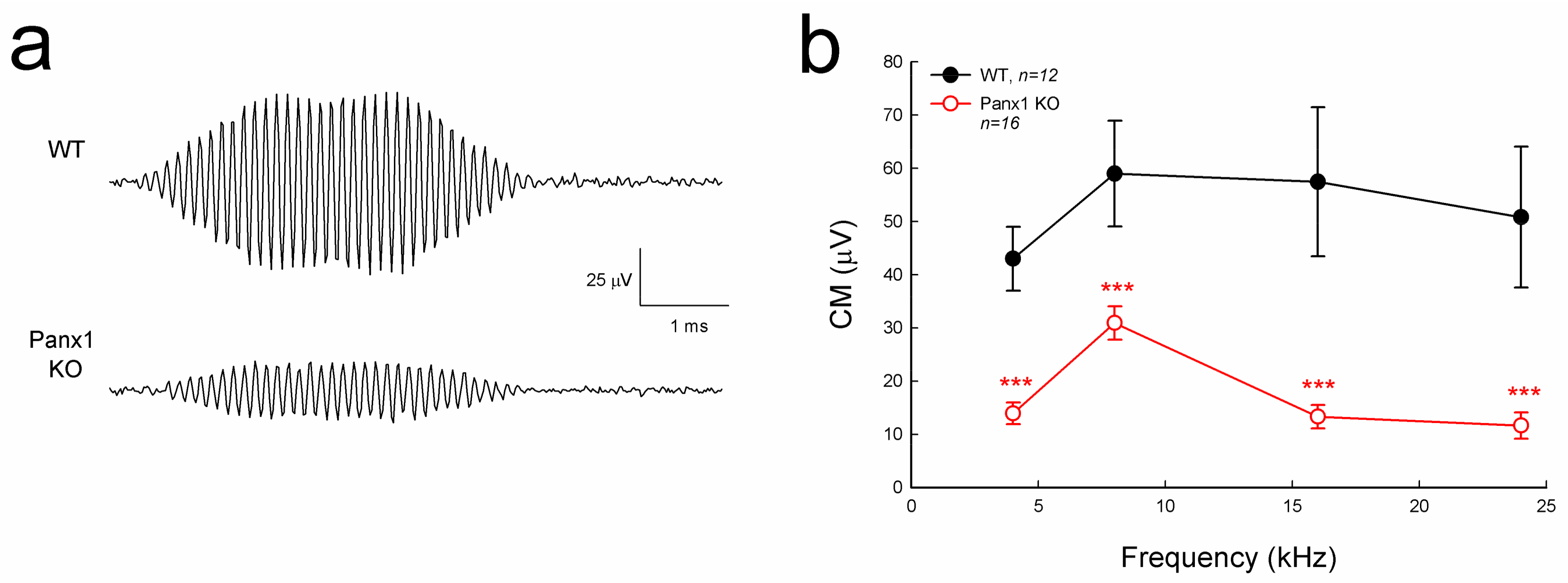
2.4. Reduction of Auditory Receptor Potential in Gen-Panx1 KO Mice
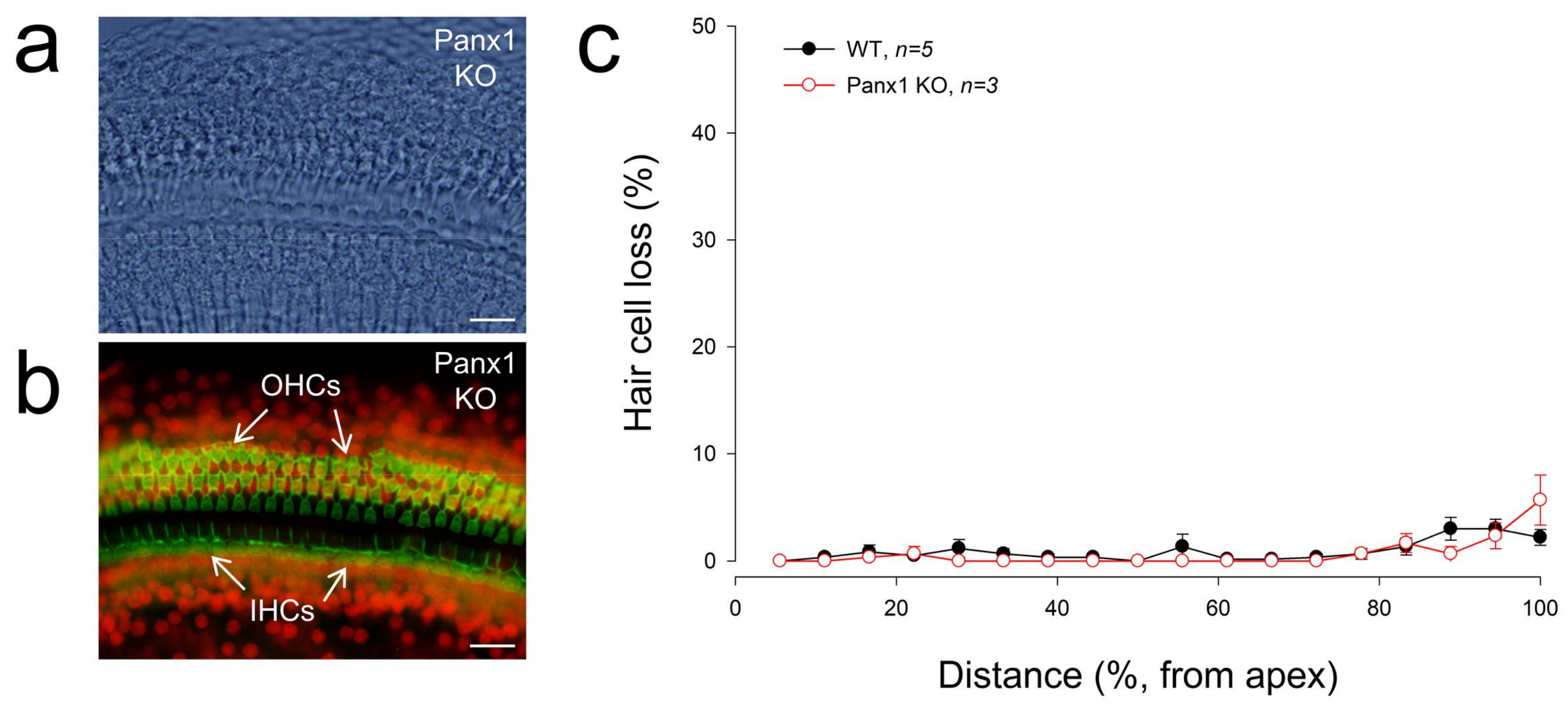
2.5. No Apparent Hair Cell Loss in Gen-Panx1 KO Mice
3. Discussion
4. Materials and Methods
4.1. Panx1 KO Mice and Genotyping
4.2. Data Processing and Statistical Analysis
4.3. ABR, CM, and DPOAE Recordings
4.4. Cochlear Preparation and Immunofluorescent Staining
4.5. Cochlear Epithelium Whole-Mounting and Hair Cell Loss Accounting
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | auditory brainstem response |
| CM | cochlear microphonics |
| cKO | conditional knockout |
| DPOAE | distortion product otoacoustic emission |
| EMMA | European Mouse Mutant Archive |
| EP | endocochlear potential |
| KO | knockout |
| OC | organ of Corti |
| OHC | outer hair cell |
| OSC | outer sulcus cell |
| PTS | permanent threshold shift |
| SLM | spiral limbus |
| WT | wild-type |
References
- Castillo, F.J.; Castillo, I. The DFNB1 subtype of autosomal recessive non-syndromic hearing impairment. Front. Biosci. 2011, 17, 3252–3274. [Google Scholar] [CrossRef]
- Castillo, F.J.; Castillo, I. DFNB1 non-syndromic hearing impairment: Diversity of mutations and associated phenotypes. Front. Mol. Neurosci. 2017, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.; Chang, K.W. GJB2-associated hearing loss: Systematic review of worldwide prevalence, genotype, and auditory phenotype. Laryngoscope 2014, 124, E34–E53. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.C.; Zhao, H.B. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss—A Common Hereditary Deafness. Front. Cell. Neurosci. 2015, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B. Hypothesis of K+-recycling defect is not a primary deafness mechanism for Cx26 (GJB2) deficiency. Front. Mol. Neurosci. 2017, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, R.; Hormuzdi, S.G.; Barbe, M.T.; Herb, A.; Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13644–13649. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Ivanov, D.; Petrash, N.; Pestova, A.; Skoblov, M.; Kelmanson, I.; Shagin, D.; Nazarenko, S.; Geraymovych, E.; Litvin, O.; et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 2004, 83, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Streeter, M.; Liu, Y.P.; Zhao, H.B. Identification and characterization of pannexin expression in the mammalian cochlea. J. Comp. Neurol. 2009, 512, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Bao, L.; Dahl, G. Pannexin 1 in erythrocytes: Function without a gap. Proc. Natl. Acad. Sci. USA 2006, 103, 7655–7659. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011, 5, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G. ATP release through pannexon channels. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 1672. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Zhou, N.; MacVicar, B.A. Ischemia opens neuronal gap junction hemichannels. Science 2006, 312, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Jackson, M.F.; Olah, M.E.; Rungta, R.L.; Hines, D.J.; Beazely, M.A.; MacDonald, J.F.; MacVicar, B.A. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 2008, 322, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Misaghi, S.; Newton, K.; Gilmour, L.L.; Louie, S.; Cupp, J.E.; Dubyak, G.R.; Hackos, D.; Dixit, V.M. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 2011, 186, 6553–6561. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Gehi, R.; Laird, D.W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta 2013, 1828, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Kelly, J.J.; Churko, J.M.; Barr, K.J.; Berger, A.C.; Laird, D.W. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J. Investig. Dermatol. 2014, 134, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.; Chiu, Y.H.; Armstrong, A.J.; Kinchen, J.M.; Juncadella, I.J.; Bayliss, D.A.; Ravichandran, K.S. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 2014, 507, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B. Expression and function of pannexins in the inner ear and hearing. BMC Cell Biol. 2016, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Liang, C.; Chen, J.; Zhao, H.B. Pannexin1 channels dominate ATP release in the cochlea ensuring endocochlear potential and auditory receptor potential generation and hearing. Sci. Rep. 2015, 5, 10762. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Bringmann, A.; Reichenbach, A. Purinergic signaling in special senses. Trends Neurosci. 2009, 32, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Yu, N.; Fleming, C.R. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. USA 2005, 102, 18724–18729. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Zhao, H.B. ATP activates P2x receptors and requires extracellular Ca++ participation to modify outer hair cell nonlinear capacitance. Pflugers Arch. 2008, 457, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Morton-Jones, R.; Vlajkovic, S.M.; Telang, R.S.; Paramananthasivam, V.; Tadros, S.F.; Wong, A.C.; Froud, K.E.; Cederholm, J.M.; Sivakumaran, Y.; et al. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc. Natl. Acad. Sci. USA 2013, 110, 7494–7499. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, N.X.; Yi, E.; Gale, J.E.; Glowatzki, E.; Bergles, D.E. The origin of spontaneous activity in the developing auditory system. Nature 2007, 450, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, N.X.; Bergles, D.E. Developmental regulation of spontaneous activity in the mammalian cochlea. J. Neurosci. 2010, 30, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, H.B. ATP activates P2X receptors to mediate gap junctional coupling in the cochlea. Biochem. Biophys. Res. Commun. 2012, 426, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, H.B. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signal. 2010, 6, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Zhu, Y.; Liang, C.; Chen, J. Pannexin 1 deficiency can induce hearing loss. Biochem. Biophys. Res. Commun. 2015, 463, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Lindstrom, K.; Shi, R.; Kelly, J.; Schroeder, A.; Juusola, J.; Levine, K.L.; Esseltine, J.L.; Penuela, S.; Jackson, M.F.; et al. A germline variant in the PANX1 gene has reduced channel function and is associated with multisystem dysfunction. J. Biol. Chem. 2016, 291, 12432–12443. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, F.; Hernandez, V.H.; Crispino, G.; Seydel, A.; Ortolano, S.; Roper, S.D.; Kessaris, N.; Richardson, W.; Rickheit, G.; Filippov, M.A.; et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 18770–18775. [Google Scholar] [CrossRef] [PubMed]
- Hanstein, R.; Negoro, H.; Patel, N.K.; Charollais, A.; Meda, P.; Spray, D.C.; Suadicani, S.O.; Scemes, E. Promises and pitfalls of a Pannexin1 transgenic mouse line. Front. Pharmacol. 2013, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, J.M.; Kelly, J.J.; Barr, K.; Schormans, A.L.; Laird, D.W.; Allman, B.L. Differential effects of pannexins on noise-induced hearing loss. Biochem. J. 2016, 473, 4665–4680. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, V.; Paciello, F.; Ziraldo, G.; Peres, C.; Mazzarda, F.; Nardin, C.; Pasquini, M.; Chiani, F.; Raspa, M.; Scavizzi, F.; et al. Mouse Panx1 is dispensable for hearing acquisition and auditory function. Front. Mol. Neurosci. 2017, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Bargiotas, P.; Krenz, A.; Hormuzdi, S.G.; Ridder, D.A.; Herb, A.; Barakat, W.; Penuela, S.; von Engelhardt, J.; Monyer, H.; Schwaninger, M. Pannexins in ischemia-induced neurodegeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 20772–20777. [Google Scholar] [CrossRef] [PubMed]
- Dvoriantchikova, G.; Ivanov, D.; Panchin, Y.; Shestopalov, V.I. Expression of pannexin family of proteins in the retina. FEBS Lett. 2006, 580, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- Zoidl, G.; Petrasch-Parwez, E.; Ray, A.; Meier, C.; Bunse, S.; Habbes, H.W.; Dahl, G.; Dermietzel, R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 2007, 146, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, H.B. The role of an inwardly rectifying K+ channel (Kir4.1) in the inner ear and hearing loss. Neuroscience 2014, 265, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Kimura, R.S.; Paul, D.L.; Adams, J.C. Gap junctions in the rat cochlea: Immunohistochemical and ultrastructural analysis. Anat. Embryol. 1995, 191, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Yu, N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J. Comp. Neurol. 2006, 499, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhu, Y.; Zong, L.; Lu, G.J.; Zhao, H.B. Cell degeneration is not a primary causer for Connexin26 (GJB2) deficiency associated hearing loss. Neurosci. Lett. 2012, 528, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Zhu, Y.; Liang, C.; Zhao, H.B. Deafness induced by Connexin26 (GJB2) deficiency is not determined by endocochlear potential (EP) reduction but is associated with cochlear developmental disorders. Biochem. Biophys. Res. Commun. 2014, 448, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, C.; Chen, J.; Zong, L.; Chen, G.D.; Zhao, H.B. Active cochlear amplification is dependent on supporting cell gap junctions. Nat. Commun. 2013, 4, 1786. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, J.; Liang, C.; Zong, L.; Chen, J.; Jones, R.O.; Zhao, H.B. Connexin26 (GJB2) deficiency reduces active cochlear amplification leading to late-onset hearing loss. Neuroscience 2015, 284, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Chen, J.; Zong, L.; Zhu, Y.; Liang, C.; Jones, R.O.; Zhao, H.B. A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: Reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall. Neurobiol. Dis. 2017, 108, 195–203. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liang, C.; Zong, L.; Zhu, Y.; Zhao, H.-B. Knockout of Pannexin-1 Induces Hearing Loss. Int. J. Mol. Sci. 2018, 19, 1332. https://doi.org/10.3390/ijms19051332
Chen J, Liang C, Zong L, Zhu Y, Zhao H-B. Knockout of Pannexin-1 Induces Hearing Loss. International Journal of Molecular Sciences. 2018; 19(5):1332. https://doi.org/10.3390/ijms19051332
Chicago/Turabian StyleChen, Jin, Chun Liang, Liang Zong, Yan Zhu, and Hong-Bo Zhao. 2018. "Knockout of Pannexin-1 Induces Hearing Loss" International Journal of Molecular Sciences 19, no. 5: 1332. https://doi.org/10.3390/ijms19051332



