Sphingosine-1-Phosphate Receptor 1 Is Involved in Non-Obese Diabetic Mouse Thymocyte Migration Disorders
Abstract
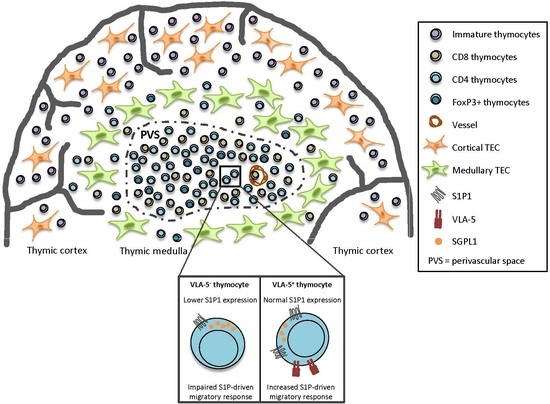
:1. Introduction
2. Results
2.1. Decreased S1P1 Expression in NOD Thymocytes
2.2. Decreased Expression of S1P Lyase 1 in NOD Thymocytes
2.3. CD49e-Negative NOD Mouse Thymocytes Have Impaired S1P-Driven Migratory Response
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibodies and Chemicals
4.3. Flow Cytometry
4.4. Real-Time Quantitative Polymerase Chain Reaction (Quantitative RT-PCR)
4.5. Immunohistochemistry
4.6. Transmigration Assays
4.7. S1P Quantitation
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NOD | Non-Obese Diabetic |
| VLA | Very Late Antigen |
| S1P | Sphingosine-1-Phosphate |
| S1P1 | Sphingosine-1-Phosphate receptor 1 |
| T1D | Type I Diabetes |
| PVS | Perivascular Spaces |
| ECM | Extracellular Matrix |
| SGPL1 | S1P lyase 1 |
References
- Anderson, M.S.; Bluestone, J.A. The NOD Mouse: A Model of Immune Dysregulation. Annu. Rev. Immunol. 2005, 23, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yui, R.; Kusumoto, Y.; Serizawa, Y.; Makino, S.; Tochino, Y. Lymphocytic insulitis in a ‘non-obese diabetic (NOD)’ strain of mice: An immunohistochemical and electron microscope investigation. Biomed. Res. 1982, 3, 429–443. [Google Scholar] [CrossRef]
- Ogawa, M.; Maruyamai, T.; Hasegawai, T.; Kanaya, T.; Kobayashii, F.; Tochino, Y.; Uda, H. The inhibitory effect of neonatal thymectomy on the incidence of insulitis in non-obese diabetes (NOD) mice. Biomed. Res. 1985, 6, 103–105. [Google Scholar] [CrossRef]
- Savino, W.; Mendes-Da-Cruz, D.A.; Silva, J.S.; Dardenne, M.; Cotta-De-Almeida, V. Intrathymic T-cell migration: A combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 2002, 23, 305–313. [Google Scholar] [CrossRef]
- Savino, W.; Boitard, C.; Bach, J.-F.; Dardenne, M. Studies on the thymus in nonobese diabetic mouse. I. Changes in the microenvironmental compartments. Lab. Investig. 1991, 63, 405–417. [Google Scholar]
- Mendes-da-Cruz, D.A.; Smaniotto, S.; Keller, A.C.; Dardenne, M.; Savino, W. Multivectorial abnormal cell migration in the NOD mouse thymus. J. Immunol. 2008, 180, 4639–4647. [Google Scholar] [CrossRef] [PubMed]
- Cotta-de-Almeida, V.; Serra Villa-Verde, D.M.; Lepault, F.; Pléau, J.-M.; Dardenne, M.; Savino, W. Impaired migration of NOD mouse thymocytes: A fibronectin receptor-related defect. Eur. J. Immunol. 2004, 34, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Geutskens, S.B.; Mendes-da-Cruz, D.A.; Dardenne, M.; Savino, W. Fibronectin receptor defects in NOD mouse leucocytes: Possible consequences for type 1 diabetes. Scand. J. Immunol. 2004, 60, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007, 115, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, M.A.; Cyster, J.G. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 2010, 328, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.-W.; Huang, Y.; Cyster, J.G.; et al. Promotion of Lymphocyte Egress into Blood and Lymph by Distinct Sources of Sphingosine-1-Phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Dreier, J.L.; Mandala, S.; Proia, R.L. Expression of the Sphingosine-1-Phosphate Receptor, S1P1, on T-cells Controls Thymic Emigration. J. Biol. Chem. 2004, 279, 15396–15401. [Google Scholar] [CrossRef] [PubMed]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine-1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; An, S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine-1-phosphate. FASEB J. 1998, 12, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Contos, J.J.A.; Weiner, J.A.; Fukushima, N.; Chun, J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene 1999, 227, 89–99. [Google Scholar] [CrossRef]
- Graeler, M.; Shankar, G.; Goetzl, E.J. Cutting Edge: Suppression of T Cell Chemotaxis by Sphingosine-1-Phosphate. J. Immunol. 2002, 169, 4084–4087. [Google Scholar] [CrossRef] [PubMed]
- Messias, C.V.; Santana-Van-Vliet, E.; Lemos, J.P.; Moreira, O.C.; Cotta-de-Almeida, V.; Savino, W.; Mendes-da-Cruz, D.A. Sphingosine-1-Phosphate Induces Dose-Dependent Chemotaxis or Fugetaxis of T-ALL Blasts through S1P1 Activation. PLoS ONE 2016, 11, e0148137. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Blaho, V.A.; Hla, T.; Han, M.H. Sphingosine-1-phosphate receptor 1 signalling in T cells: Trafficking and beyond. Immunology 2014, 142, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Don, A.S.; Lim, X.Y.; Couttas, T.A. Re-Configuration of Sphingolipid Metabolism by Oncogenic Transformation. Biomolecules 2014, 4, 315–353. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Kunimoto, K.; Muraoka, Y.; Mizushima, Y.; Katagiri, K.; Tochino, Y. Breeding of a non-obese, diabetic strain of mice. Exp. Anim. 1980, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Kunimoto, K.; Muraoka, Y.; Katagiri, K. Effect of castration on the appearance of diabetes in NOD mouse. Exp. Anim. 1981, 30, 137–140. [Google Scholar] [CrossRef]
- Colomb, E.; Savino, W.; Wicker, L.; Peterson, L.; Dardenne, M.; Carnaud, C. Genetic control of giant perivascular space formation in the thymus of NOD mice. Diabetes 1996, 45, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Bergerot, I.; Elliott, J.F.; Harrison, L.C.; Abiru, N.; Eisenbarth, G.S.; Delovitch, T.L. Evidence that a peptide spanning the B–C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J. Immunol. 2001, 167, 4926–4935. [Google Scholar] [CrossRef] [PubMed]
- Brimnes, M.K.; Jensen, T.; Jørgensen, T.N.; Michelsen, B.K.; Troelsen, J.; Werdelin, O. Low expression of insulin in the thymus of non-obese diabetic mice. J. Autoimmun. 2002, 19, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Saba, J.D.; Hla, T. Point-Counterpoint of Sphingosine-1-Phosphate Metabolism. Circ. Res. 2004, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, A.D.; Bandhuvula, P.; Kumar, A.; Yoshinaga, Y.; Nefedov, M.; Fong, L.G.; Zhang, M.; Baridon, B.; Dillard, L.; de Jong, P.; et al. Sphingosine-1-phosphate lyase expression in embryonic and adult murine tissues. J. Lipid Res. 2012, 53. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Yagi, H.; Takemoto, K.; Utsumi, H.; Fukunari, A.; Sugahara, K.; Masuko, T.; Chiba, K. S1P lyase in thymic perivascular spaces promotes egress of mature thymocytes via up-regulation of S1P receptor 1. Int. Immunol. 2013, 26, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte Sequestration Through S1P Lyase Inhibition and Disruption of S1P Gradients. Science 2005, 309, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Bunting, M.D.; Comerford, I.; McColl, S.R. Finding their niche: Chemokines directing cell migration in the thymus. Immunol. Cell Biol. 2011, 89, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Yatomi, Y.; Shimosawa, T.; Yamashita, H.; Kitayama, J.; Tsuno, N.H.; Takahashi, K.; Ozaki, Y. The suppressive effect of sphingosine-1-phosphate on monocyte-endothelium adhesion may be mediated by the rearrangement of the endothelial integrins α5β1 and αvβ3. J. Thromb. Haemost. 2007, 5, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, J.-F.; Lin, C.-Y.; Lee, M.-J. Rho GTPases mediated integrin αvβ3 activation in sphingosine-1-phosphate stimulated chemotaxis of endothelial cells. Histochem. Cell Biol. 2008, 129, 579–588. [Google Scholar] [CrossRef] [PubMed]
- García-Bernal, D.; Redondo-Muñoz, J.; Dios-Esponera, A.; Chèvre, R.; Bailón, E.; Garayoa, M.; Arellano-Sánchez, N.; Gutierrez, C.N.; Hidalgo, A.; García-Pardo, A.; et al. Sphingosine-1-phosphate activates chemokine-promoted myeloma cell adhesion and migration involving α4β1 integrin. J. Pathol. 2013, 229, 36–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. 2002, 3, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Lin Oo, M.; Thangada, S.; Wu, M.T.; Liu, C.H.; Macdonald, T.L.; Lynch, K.R.; Lin, C.Y.; Hla, T. Immunosuppressive and anti-angiogenic sphingosine-1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 2007, 282, 9082–9089. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.R.; Cyster, J.G. Finding a way out: Lymphocyte egress from lymphoid organs. Nat. Immunol. 2007, 8, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Pineda, J.; Kumar, A.; Suh, J.H.; Zhang, M.; Saba, J.D. Dendritic cell sphingosine-1-phosphate lyase regulates thymic egress. J. Exp. Med. 2016, 213, 2773–2791. [Google Scholar] [CrossRef] [PubMed]
- Einicker-Lamas, M.; Wenceslau, L.D.; Bernardo, R.R.; Nogaroli, L.; Guilherme, A.; Oliveira, M.M.; Vieyra, A. Sphingosine-1-Phosphate Formation Activates Phosphatidylinositol-4 Kinase in Basolateral Membranes from Kidney Cells: Crosstalk in Cell Signaling through Sphingolipids and Phospholipids. J. Biochem. 2003, 134, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Lepletier, A.; De Almeida, L.; Santos, L.; Sampaio, S.; Paredes, B.; Bele, F.; Savino, W.; Morrot, A.; Einicker-lamas, M.; Pe, A.R. Early Double-Negative Thymocyte Export in Trypanosoma cruzi Infection Is Restricted by Sphingosine Receptors and Associated with Human Chagas Disease. PLoS Negl. Trop. Dis. 2014, 8, e3203. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemos, J.P.; Smaniotto, S.; Messias, C.V.; Moreira, O.C.; Cotta-de-Almeida, V.; Dardenne, M.; Savino, W.; Mendes-da-Cruz, D.A. Sphingosine-1-Phosphate Receptor 1 Is Involved in Non-Obese Diabetic Mouse Thymocyte Migration Disorders. Int. J. Mol. Sci. 2018, 19, 1446. https://doi.org/10.3390/ijms19051446
Lemos JP, Smaniotto S, Messias CV, Moreira OC, Cotta-de-Almeida V, Dardenne M, Savino W, Mendes-da-Cruz DA. Sphingosine-1-Phosphate Receptor 1 Is Involved in Non-Obese Diabetic Mouse Thymocyte Migration Disorders. International Journal of Molecular Sciences. 2018; 19(5):1446. https://doi.org/10.3390/ijms19051446
Chicago/Turabian StyleLemos, Julia P., Salete Smaniotto, Carolina V. Messias, Otacilio C. Moreira, Vinicius Cotta-de-Almeida, Mireille Dardenne, Wilson Savino, and Daniella A. Mendes-da-Cruz. 2018. "Sphingosine-1-Phosphate Receptor 1 Is Involved in Non-Obese Diabetic Mouse Thymocyte Migration Disorders" International Journal of Molecular Sciences 19, no. 5: 1446. https://doi.org/10.3390/ijms19051446