Lambertianic Acid Sensitizes Non-Small Cell Lung Cancers to TRAIL-Induced Apoptosis via Inhibition of XIAP/NF-κB and Activation of Caspases and Death Receptor 4
Abstract
:1. Introduction
2. Results
2.1. Combined Treatment of LA and TRAIL Enhanced Cytotoxicity in A549 and H1299 Non-Small Cell Lung Cancer Cells
2.2. Combined Treatment of LA and TRAIL Significantly Increased the Sub-G1 Population and Also Increased the Cleavage of PARP and Caspase8/9/3 in A549 and H1299 Non-Small Cell Lung Cancer Cells
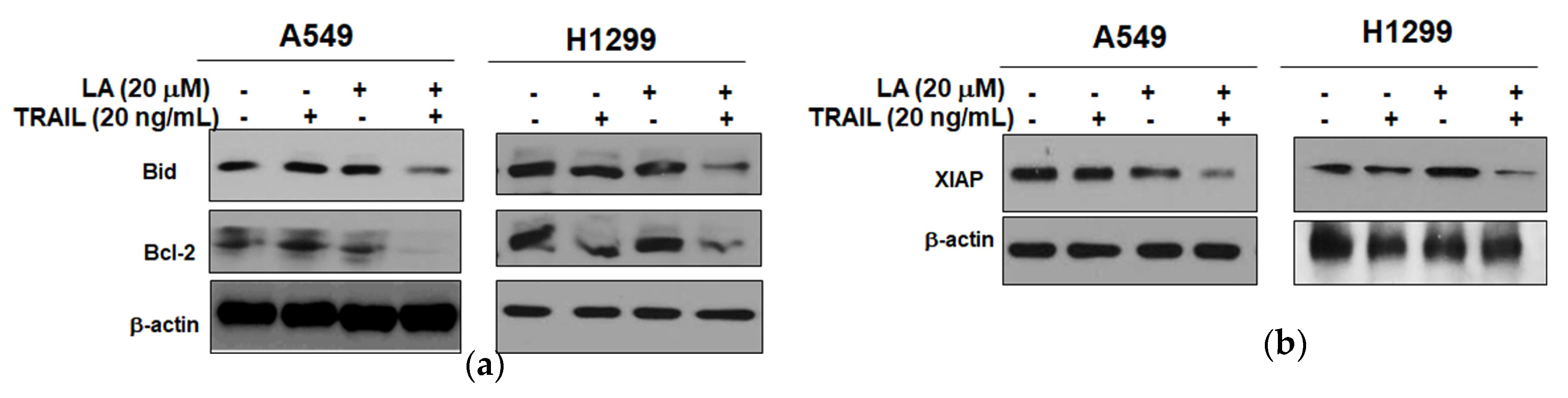
2.3. Combined Treatment of LA and TRAIL Regulated Antiapoptotic and Proapoptotic Proteins in A549 and H1299 Non-Small Cell Lung Cancer Cells
2.4. Combined Treatment of LA and TRAIL Upregulated the Expression of DR4 and Inhibited the Expression of p-NF-κB, p-IκB, and FLIP in A549 and H1299 Non-Small Cell Lung Cancer Cells
2.5. Combined Treatment of LA and TRAIL Disrupted Binding of XIAP with Caspase3 and NF-κB in A549 Non-Small Cell Lung Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Lambertianic Acid Isolation
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Crystal Violet Assay
4.5. Cell Cycle Analysis
4.6. Western Blotting
4.7. Co-Immunoprecipitation
4.8. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TRAIL | TNF-related apoptosis-inducing ligand |
| PARP | Poly (ADP-ribose) polymerase |
| Caspase | Cysteine aspartyl-specific protease |
| Bcl-2 | B-cell lymphoma 2 |
| XIAP | X-linked inhibitor of apoptosis protein |
| DR5 | Death receptor5 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Secchiero, P.; Vaccarezza, M.; Gonelli, A.; Zauli, G. TNF-related apoptosis-inducing ligand (TRAIL): A potential candidate for combined treatment of hematological malignancies. Curr. Pharm. Des. 2004, 10, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008698. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.; Abd-Rabou, A.; Reda, A. TRAIL combinations: The new ‘trail’ for cancer therapy (Review). Oncol. Lett. 2014, 7, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Parajuli, K.R.; Han, S.I. The alkyllysophospholipid edelfosine enhances TRAIL-mediated apoptosis in gastric cancer cells through death receptor 5 and the mitochondrial pathway. Tumour Biol. 2016, 37, 6205–6216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yun, M.; Kim, E.O.; Jung, D.B.; Won, G.; Kim, B.; Jung, J.H.; Kim, S.H. Decursin enhances TRAIL-induced apoptosis through oxidative stress mediated- endoplasmic reticulum stress signalling in non-small cell lung cancers. Br. J. Pharmacol. 2016, 173, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, B.; Wang, Y.; Ding, H. Kaempferol Sensitizes Human Ovarian Cancer Cells-OVCAR-3 and SKOV-3 to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis via JNK/ERK-CHOP Pathway and Up-Regulation of Death Receptors 4 and 5. Med. Sci. Monit. 2017, 23, 5096–5105. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Um, H.J.; Seo, S.U.; Woo, S.M.; Kim, S.; Park, J.W.; Lee, H.S.; Kim, S.H.; Choi, Y.H.; Lee, T.J.; et al. Angelicin potentiates TRAIL-induced apoptosis in renal carcinoma Caki cells through activation of caspase 3 and down-regulation of c-FLIP expression. Drug Dev. Res. 2018, 79, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Z.; Wu, Q.; Chen, S.; Yi, C.; Gong, C. TRAIL and curcumin codelivery nanoparticles enhance TRAIL-induced apoptosis through upregulation of death receptors. Drug Deliv. 2017, 24, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Cho, S.M.; Lee, M.H.; Lee, E.O.; Kim, S.H.; Lee, H.J. Ethanol extract of Pinus koraiensis leaves containing lambertianic acid exerts anti-obesity and hypolipidemic effects by activating adenosine monophosphate-activated protein kinase (AMPK). BMC Complement. Altern. Med. 2016, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Chin, Y.W. Anti-allergic effect of lambertianic acid from Thuja orientalis in mouse bone marrow-derived mast cells. Immunopharmacol. Immunotoxicol. 2012, 34, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Jeong, A.; Kim, J.H.; Lee, H.J.; Kim, S.H. Reactive oxygen species dependent phosphorylation of the liver kinase B1/AMP activated protein kinase/acetyl-CoA carboxylase signaling is critically involved in apoptotic effect of lambertianic acid in hepatocellular carcinoma cells. Oncotarget 2017, 8, 70116–70129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene 2008, 27, 6207–6215. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Griffith, T.S. The role of Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) in the ocular immune response. Chem. Immunol. Allergy 2007, 92, 140–154. [Google Scholar] [PubMed]
- Trivedi, R.; Mishra, D.P. Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, L.Y.; Anderson, C.K.; Camidge, R.; Behbakht, K.; Thorburn, A.; Ford, H.L. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene 2013, 32, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lu, G.D.; Ong, C.S.; Ong, C.N.; Shen, H.M. Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis via p53-mediated death receptor 4 up-regulation. Mol. Cancer Ther. 2008, 7, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Zhong, M.Z.; Yuan, G.J.; Hou, S.P.; Yin, L.L.; Jiang, H.; Yu, Z. Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol. Rep. 2013, 29, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Baritaki, S.; Suzuki, E.; Umezawa, K.; Spandidos, D.A.; Berenson, J.; Daniels, T.R.; Penichet, M.L.; Jazirehi, A.R.; Palladino, M.; Bonavida, B. Inhibition of Yin Yang 1-dependent repressor activity of DR5 transcription and expression by the novel proteasome inhibitor NPI-0052 contributes to its TRAIL-enhanced apoptosis in cancer cells. J. Immunol. 2008, 180, 6199–6210. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Suzuki, S.; Koizumi, K.; Prangsaengtong, O.; Viriyaroj, A.; Ruchirawat, S.; Svasti, J.; Saiki, I. Vanillin enhances TRAIL-induced apoptosis in cancer cells through inhibition of NF-κB activation. In Vivo 2010, 24, 501–506. [Google Scholar] [PubMed]
- Franco, A.V.; Zhang, X.D.; Van Berkel, E.; Sanders, J.E.; Zhang, X.Y.; Thomas, W.D.; Nguyen, T.; Hersey, P. The role of NF-κ B in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J. Immunol. 2001, 166, 5337–5345. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, X.; Li, J.; Luo, W.; Huang, C.; Chen, J. X-linked inhibitor of apoptosis protein (XIAP) lacking RING domain localizes to the nuclear and promotes cancer cell anchorage-independent growth by targeting the E2F1/Cyclin E axis. Oncotarget 2014, 5, 7126–7137. [Google Scholar] [CrossRef] [PubMed]
- Liston, P.; Fong, W.G.; Korneluk, R.G. The inhibitors of apoptosis: There is more to life than Bcl2. Oncogene 2003, 22, 8568–8580. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lin, S.C.; Huang, Y.; Kang, Y.J.; Rich, R.; Lo, Y.C.; Myszka, D.; Han, J.; Wu, H. XIAP induces NF-κB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell 2007, 26, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Gyrd-Hansen, M.; Meier, P. IAPs: From caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat. Rev. Cancer 2010, 10, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.D.; Yu, J.; Chi, J.L.; Long, F.W.; Yang, H.W.; Chen, K.L.; Lv, Z.Y.; Zhou, B.; Peng, Z.H.; et al. Deletion Of XIAP reduces the severity of acute pancreatitis via regulation of cell death and nuclear factor-κB activity. Cell Death Dis. 2017, 8, e2685. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ding, Y.; Sun, Z.H.; Zhang, D.M. Studies on chemical constituents of Pinus armandii. Yao Xue Xue Bao 2005, 40, 435–437. [Google Scholar] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, D.S.; Lee, H.J.; Hwang, J.; Han, H.; Kim, B.; Shim, B.; Kim, S.-H. Lambertianic Acid Sensitizes Non-Small Cell Lung Cancers to TRAIL-Induced Apoptosis via Inhibition of XIAP/NF-κB and Activation of Caspases and Death Receptor 4. Int. J. Mol. Sci. 2018, 19, 1476. https://doi.org/10.3390/ijms19051476
Ahn DS, Lee HJ, Hwang J, Han H, Kim B, Shim B, Kim S-H. Lambertianic Acid Sensitizes Non-Small Cell Lung Cancers to TRAIL-Induced Apoptosis via Inhibition of XIAP/NF-κB and Activation of Caspases and Death Receptor 4. International Journal of Molecular Sciences. 2018; 19(5):1476. https://doi.org/10.3390/ijms19051476
Chicago/Turabian StyleAhn, Deok Soo, Hyo Jung Lee, Jisung Hwang, Hyukgyu Han, Bonglee Kim, BumSang Shim, and Sung-Hoon Kim. 2018. "Lambertianic Acid Sensitizes Non-Small Cell Lung Cancers to TRAIL-Induced Apoptosis via Inhibition of XIAP/NF-κB and Activation of Caspases and Death Receptor 4" International Journal of Molecular Sciences 19, no. 5: 1476. https://doi.org/10.3390/ijms19051476
APA StyleAhn, D. S., Lee, H. J., Hwang, J., Han, H., Kim, B., Shim, B., & Kim, S.-H. (2018). Lambertianic Acid Sensitizes Non-Small Cell Lung Cancers to TRAIL-Induced Apoptosis via Inhibition of XIAP/NF-κB and Activation of Caspases and Death Receptor 4. International Journal of Molecular Sciences, 19(5), 1476. https://doi.org/10.3390/ijms19051476







