Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer
Abstract
:1. Introduction
2. The CCL5/CCR5 Axis in Cancer: General Mechanisms
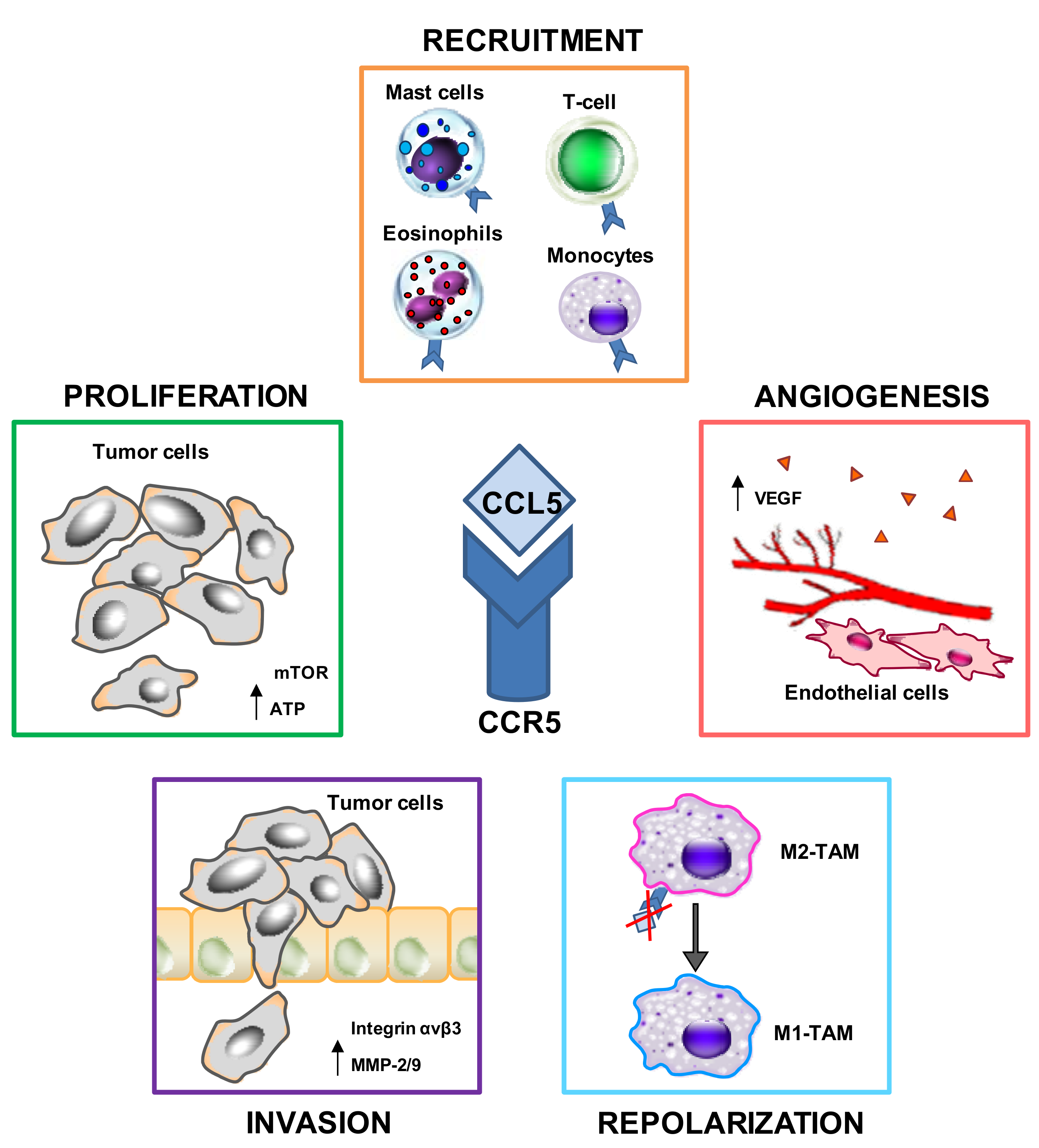
2.1. CCL5–CCR5 Interactions May Favor Tumor Development in Multiple Ways
2.1.1. Proliferation
2.1.2. Immunosuppression
2.1.3. Angiogenesis
2.1.4. Migration (Metastasis Formation)
3. Possible Clinical Applications: CCL5 and CCR5 as Therapeutic Targets
3.1. Inhibition of CCL5–CCR5 Interactions
3.2. Inhibition of CCL5 Secretion
4. Gastric Cancer and Its TME
4.1. Macrophages (TAMs)
4.2. Regulatory T Cells (T-Regs)
4.3. Cancer-Associated Fibroblasts (CAFs)
4.4. Endothelial Cells (Angiogenesis)
5. The CCL5/CCR5 Axis in GC Development and/or Progression
6. Possible Clinical Applications of MVC in GC
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mantovani, A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010, 10, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Westwood, J.A.; Darcy, P.K. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer 2013, 13, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, T.G. Dual role of inflammatory mediators in cancer. Ecancermedicalscience 2017, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Hagemann, T. Tumour-associated macrophages and cancer. Curr. Opin. Pharmacol. 2013, 13, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Lin, Y.C.; Mahalingam, J.; Huang, C.T.; Chen, T.W.; Kang, C.W.; Peng, H.M.; Chu, Y.Y.; Chiang, J.M.; Dutta, A.; et al. Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012, 72, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hou, J.; Han, Z.; Wang, Y.; Hao, C.; Wei, L.; Shi, Y. One cell, multiple roles: Contribution of mesenchymal stem cells to tumor development in tumor microenvironment. Cell Biosci. 2013, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Schlecker, E.; Stojanovic, A.; Eisen, C.; Quack, C.; Falk, C.S.; Umansky, V.; Cerwenka, A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 2012, 189, 5602–5611. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Germano, G.; Marchesi, F.; Mantovani, A. Chemokines in cancer related inflammation. Exp. Cell Res. 2011, 317, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Wilber, A. Roles of inflammation in cancer initiation, progression, and metastasis. Front. Biosci. 2010, 2, 176–183. [Google Scholar]
- Balkwill, F.R. The chemokine system and cancer. J. Pathol. 2012, 226, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Lorenzon, D.; Cattaruzza, L.; Pinto, A.; Gloghini, A.; Carbone, A.; Colombatti, A. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: Involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int. J. Cancer 2008, 122, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Celegato, M.; Casagrande, N. Microenvironmental interactions in classical Hodgkin lymphoma and their role in promoting tumor growth, immune escape and drug resistance. Cancer Lett. 2016, 380, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Meadows, S.A.; Vega, F.; Kashishian, A.; Johnson, D.; Diehl, V.; Miller, L.L.; Younes, A.; Lannutti, B.J. PI3Kdelta inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood 2012, 119, 1897–1900. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Colombatti, A. The inflammatory chemokine CCL5 and cancer progression. Mediat. Inflamm. 2014, 2014, 292376. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Aldinucci, D.; Gloghini, A.; Pinto, A.; Colombatti, A.; Carbone, A. The role of CD40/CD40L and interferon regulatory factor 4 in Hodgkin lymphoma microenvironment. Leuk. Lymphoma 2012, 53, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Udi, J.; Schuler, J.; Wider, D.; Ihorst, G.; Catusse, J.; Waldschmidt, J.; Schnerch, D.; Follo, M.; Wasch, R.; Engelhardt, M. Potent in vitro and in vivo activity of sorafenib in multiple myeloma: Induction of cell death, CD138-downregulation and inhibition of migration through actin depolymerization. Br. J. Haematol. 2013, 161, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Roscic-Mrkic, B.; Fischer, M.; Leemann, C.; Manrique, A.; Gordon, C.J.; Moore, J.P.; Proudfoot, A.E.; Trkola, A. RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood 2003, 102, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, M. Chemokine receptor CCR5: Insights into structure, function, and regulation. Cell. Signal. 2004, 16, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Giesler, K.E.; Tahirovic, Y.A.; Truax, V.M.; Liotta, D.C.; Wilson, L.J. CCR5 receptor antagonists in preclinical to phase II clinical development for treatment of HIV. Expert Opin. Investig. Drugs 2016, 25, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Vaday, G.G.; Peehl, D.M.; Kadam, P.A.; Lawrence, D.M. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate 2006, 66, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Murooka, T.T.; Rahbar, R.; Fish, E.N. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem. Biophys. Res. Commun. 2009, 387, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.F.; Fish, E.N. 89: A role for CCL5 in breast cancer cell metabolism. Cytokine 2013, 63, 264. [Google Scholar] [CrossRef]
- Relation, T.; Dominici, M.; Horwitz, E.M. Concise Review: An (Im)Penetrable Shield: How the Tumor Microenvironment Protects Cancer Stem Cells. Stem Cells 2017, 35, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Mai, J.; Li, X.; Mitchell-Flack, M.; Zhang, T.; Zhang, L.; Chouchane, L.; Ferrari, M.; Shen, H.; Ma, X. Targeting autocrine CCL5-CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 2017, 77, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Ben-Baruch, A. The Tumor-Promoting Flow of Cells Into, Within and Out of the Tumor Site: Regulation by the Inflammatory Axis of TNFalpha and Chemokines. Cancer Microenviron. 2012, 5, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Liu, S.C.; Sun, H.L.; Huang, T.Y.; Chan, C.H.; Yang, C.Y.; Yeh, H.I.; Huang, Y.L.; Chou, W.Y.; Lin, Y.M.; et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 2015, 36, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Fong, Y.C.; Lee, C.Y.; Chen, M.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem. Pharmacol. 2009, 77, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells 2012, 30, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Fujita, Y.; Nakane, K.; Mizutani, K.; Terazawa, R.; Ehara, H.; Kanimoto, Y.; Kojima, T.; Nozawa, Y.; Deguchi, T.; et al. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine 2013, 64, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Wu, H.H.; Liu, S.C.; Wang, P.C.; Ou, W.C.; Chou, W.Y.; Shen, Y.S.; Tang, C.H. CCL5 and CCR5 interaction promotes cell motility in human osteosarcoma. PLoS ONE 2012, 7, e35101. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.M.; Daly, D.S.; Tan, R.; Marks, J.R.; Zangar, R.C. Plasma biomarker profiles differ depending on breast cancer subtype but RANTES is consistently increased. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Smeets, A.; Brouwers, B.; Hatse, S.; Laenen, A.; Paridaens, N.; Floris, G.; Vildiers, H.; Christiaens, M.R. Circulating CCL5 Levels in Patients with Breast Cancer: Is There a Correlation with Lymph Node Metastasis? ISRN Immunol. 2013, 10, 1–5. [Google Scholar] [CrossRef]
- Dehqanzada, Z.A.; Storrer, C.E.; Hueman, M.T.; Foley, R.J.; Harris, K.A.; Jama, Y.H.; Shriver, C.D.; Ponniah, S.; Peoples, G.E. Assessing serum cytokine profiles in breast cancer patients receiving a HER2/neu vaccine using Luminex technology. Oncol. Rep. 2007, 17, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Yaal-Hahoshen, N.; Shina, S.; Leider-Trejo, L.; Barnea, I.; Shabtai, E.L.; Azenshtein, E.; Greenberg, I.; Keydar, I.; Ben-Baruch, A. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin. Cancer Res. 2006, 12, 4474–4480. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Akamatsu, H.; Niwa, H.; Sumi, H.; Ozaki, Y.; Abe, A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin. Cancer Res. 2001, 7, 285–289. [Google Scholar] [PubMed]
- Tsukishiro, S.; Suzumori, N.; Nishikawa, H.; Arakawa, A.; Suzumori, K. Elevated serum RANTES levels in patients with ovarian cancer correlate with the extent of the disorder. Gynecol. Oncol. 2006, 102, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.R.; Sima, H.R.; Rafatpanah, H.; Hosseinnezhad, H.; Ghaffarzadehgan, K.; Valizadeh, N.; Mehrabi, B.M.; Hakimi, H.R.; Masoom, A.; Noorbakhsh, A.; et al. Serum chemokine ligand 5 (CCL5/RANTES) level might be utilized as a predictive marker of tumor behavior and disease prognosis in patients with gastric adenocarcinoma. J. Gastrointest. Cancer 2014, 45, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, H.; Ichikura, T.; Tsujimoto, H.; Kinoshita, M.; Morita, D.; Ono, S.; Chochi, K.; Tsuda, H.; Seki, S.; Mochizuki, H. Prognostic significance of expression of CCL5/RANTES receptors in patients with gastric cancer. J. Surg. Oncol. 2008, 97, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, M.; Mashima, T.; Kawata, N.; Wakatsuki, T.; Horiike, Y.; Matsusaka, S.; Dan, S.; Shinozaki, E.; Seimiya, H.; Mizunuma, N.; et al. Serum VEGF-A and CCL5 levels as candidate biomarkers for efficacy and toxicity of regorafenib in patients with metastatic colorectal cancer. Oncotarget 2016, 7, 34811–34823. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J.; Casella, D.P.; Burk, R.D.; Kelsey, K.T.; Holly, E.A. Inflammation, genetic polymorphisms in proinflammatory genes TNF-A, RANTES, and CCR5, and risk of pancreatic adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2006, 15, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Sukhatme, V.P.; Bouche, G. Drug repurposing in oncology—Patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015, 12, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Weir, S.J.; DeGennaro, L.J.; Austin, C.P. Repurposing approved and abandoned drugs for the treatment and prevention of cancer through public-private partnership. Cancer Res. 2012, 72, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Velasco-Velazquez, M.A.; Wang, M.; Li, Z.; Rui, H.; Peck, A.R.; Korkola, J.E.; Chen, X.; Xu, S.; DuHadaway, J.B.; et al. CCR5 governs DNA damage and breast cancer stem cell expansion. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, E.C.; Hamilton, M.J.; Young, A.; Wadsworth, B.J.; LePard, N.E.; Lee, H.N.; Firmino, N.; Collier, J.L.; Bennewith, K.L. Maraviroc decreases CCL8-mediated migration of CCR5(+) regulatory T cells and reduces metastatic tumor growth in the lungs. Oncoimmunology 2016, 5, e1150398. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Velazquez, M.; Pestell, R.G. The CCL5/CCR5 axis promotes metastasis in basal breast cancer. Oncoimmunology 2013, 2, e23660. [Google Scholar] [CrossRef] [PubMed]
- Cambien, B.; Richard-Fiardo, P.; Karimdjee, B.F.; Martini, V.; Ferrua, B.; Pitard, B.; Schmid-Antomarchi, H.; Schmid-Alliana, A. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRbeta in colorectal carcinoma. PLoS ONE 2011, 6, e28842. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Goedegebuure, P.S.; Belt, B.A.; Flaherty, B.; Sankpal, N.; Gillanders, W.E.; Eberlein, T.J.; Hsieh, C.S.; Linehan, D.C. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol. 2009, 182, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Swamydas, M.; Ricci, K.; Rego, S.L.; Dreau, D. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adhes. Migr. 2013, 7, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Scott, K.A.; Wilson, J.L.; Thompson, R.G.; Proudfoot, A.E.; Balkwill, F.R. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003, 63, 8360–8365. [Google Scholar] [PubMed]
- Sutton, A.; Friand, V.; Papy-Garcia, D.; Dagouassat, M.; Martin, L.; Vassy, R.; Haddad, O.; Sainte-Catherine, O.; Kraemer, M.; Saffar, L.; et al. Glycosaminoglycans and their synthetic mimetics inhibit RANTES-induced migration and invasion of human hepatoma cells. Mol. Cancer Ther. 2007, 6, 2948–2958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arnatt, C.K.; Zhang, F.; Wang, J.; Haney, K.M.; Fang, X. The potential role of anibamine, a natural product CCR5 antagonist, and its analogues as leads toward development of anti-ovarian cancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 5093–5097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Haney, K.M.; Richardson, A.C.; Wilson, E.; Gewirtz, D.A.; Ware, J.L.; Zehner, Z.E.; Zhang, Y. Anibamine, a natural product CCR5 antagonist, as a novel lead for the development of anti-prostate cancer agents. Bioorg. Med. Chem. Lett. 2010, 20, 4627–4630. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Fan, W.; Sun, L.; Li, F.F.; Zhao, R.P.; Zhang, L.Y.; Yu, B.Y.; Yuan, S.T. The saponin DT-13 inhibits gastric cancer cell migration through down-regulation of CCR5-CCL5 axis. Chin. J. Nat. Med. 2014, 12, 833–840. [Google Scholar] [CrossRef]
- Ren-Ping, Z.; Sen-Sen, L.; Yuan, S.T.; Yu, B.Y.; Bai, X.S.; Sun, L.; Zhang, L.Y. DT-13, a saponin of dwarf lilyturf tuber, exhibits anti-cancer activity by down-regulating C-C chemokine receptor type 5 and vascular endothelial growth factor in MDA-MB-435 cells. Chin. J. Nat. Med. 2014, 12, 24–29. [Google Scholar] [CrossRef]
- Nichols, W.G.; Steel, H.M.; Bonny, T.; Adkison, K.; Curtis, L.; Millard, J.; Kabeya, K.; Clumeck, N. Hepatotoxicity observed in clinical trials of aplaviroc (GW873140). Antimicrob. Agents Chemother. 2008, 52, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Velazquez, M.; Jiao, X.; De La Fuente, M.; Pestell, T.G.; Ertel, A.; Lisanti, M.P.; Pestell, R.G. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012, 72, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Sasaki, S.; Mukaida, N.; Baba, T. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancer-associated fibroblast accumulation. Oncotarget 2016, 7, 48335–48345. [Google Scholar] [CrossRef] [PubMed]
- Celegato, M.; Borghese, C.; Casagrande, N.; Mongiat, M.; Kahle, X.U.; Paulitti, A.; Spina, M.; Colombatti, A.; Aldinucci, D. Preclinical activity of the repurposed drug Auranofin in classical Hodgkin lymphoma. Blood 2015, 126, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Celegato, M.; Borghese, C.; Umezawa, K.; Casagrande, N.; Colombatti, A.; Carbone, A.; Aldinucci, D. The NF-kappaB inhibitor DHMEQ decreases survival factors, overcomes the protective activity of microenvironment and synergizes with chemotherapy agents in classical Hodgkin lymphoma. Cancer Lett. 2014, 349, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; De Luca, A.; Lamura, L.; Normanno, N. Zoledronic acid blocks the interaction between mesenchymal stem cells and breast cancer cells: Implications for adjuvant therapy of breast cancer. Ann. Oncol. 2012, 23, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Borghese, C.; Casagrande, N.; Pivetta, E.; Colombatti, A.; Boccellino, M.; Amler, E.; Normanno, N.; Caraglia, M.; De Rosa, G.; Aldinucci, D. Self-assembling nanoparticles encapsulating zoledronic acid inhibit mesenchymal stromal cells differentiation, migration and secretion of proangiogenic factors and their interactions with prostate cancer cells. Oncotarget 2017, 8, 42926–42938. [Google Scholar] [CrossRef] [PubMed]
- Borghese, C.; Cattaruzza, L.; Pivetta, E.; Normanno, N.; De Luca, A.; Mazzucato, M.; Celegato, M.; Colombatti, A.; Aldinucci, D. Gefitinib inhibits the cross-talk between mesenchymal stem cells and prostate cancer cells leading to tumor cell proliferation and inhibition of docetaxel activity. J. Cell. Biochem. 2013, 114, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhu, J.; Lu, D. Molecular-targeted first-line therapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2016, 7, CD011461. [Google Scholar] [CrossRef] [PubMed]
- Bergfeld, S.A.; DeClerck, Y.A. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010, 29, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Marelli, G.; Sica, A.; Vannucci, L.; Allavena, P. Inflammation as target in cancer therapy. Curr. Opin. Pharmacol. 2017, 35, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Echizen, K.; Oshima, H.; Nakayama, M.; Oshima, M. The inflammatory microenvironment that promotes gastrointestinal cancer development and invasion. Adv. Biol. Regul. 2018, S2212–S4926. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, W.R. Gastrointestinal Malignancies. Prim. Care 2017, 44, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Machado, J.C.; Figueiredo, C. Clinical relevance of Helicobacter pylori vacA and cagA genotypes in gastric carcinoma. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.; Vyas, D.; Hollis, M.; Aekka, A.; Vyas, A. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies. World J. Gastroenterol. 2015, 21, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lim, J.B. Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J. Gastroenterol. 2014, 20, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Qu, B. Expression of chemotactic factor CCL5 in gastric cancer tissue and its correlation with macrophage marker CD86. Biomed. Res. 2017, 28, 6388–6391. [Google Scholar]
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016, 76, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.L.; Chu, G.C.; Chung, L.W. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016, 380, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Frydrychowicz, M.; Boruczkowski, M.; Kolecka-Bednarczyk, A.; Dworacki, G. The Dual Role of Treg in Cancer. Scand. J. Immunol. 2017, 86, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Takeoka, T.; Urakawa, S.; Morimoto-Okazawa, A.; Kawashima, A.; Iwahori, K.; Takiguchi, S.; Nishikawa, H.; Sato, E.; Sakaguchi, S.; et al. ICOS(+) Foxp3(+) TILs in gastric cancer are prognostic markers and effector regulatory T cells associated with Helicobacter pylori. Int. J. Cancer 2017, 140, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, R.; Guan, W.; Qiao, M.; Wang, L. Roles of microRNAs in cancer associated fibroblasts of gastric cancer. Pathol. Res. Pract. 2017, 213, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.F.; Wang, R.F. Role of cancer-associated fibroblasts in invasion and metastasis of gastric cancer. World J. Gastroenterol. 2015, 21, 9717–9726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Peng, C. Cancer-associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer. Oncol. Lett. 2018, 15, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, N.; Chai, N.; Liu, X.; Jiang, H.; Wu, Q.; Li, Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 2016, 16, 321–2351. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, G.; Gadaleta, C.D.; Zuccala, V.; Albayrak, E.; Patruno, R.; Milella, P.; Sacco, R.; Ammendola, M.; Ranieri, G. Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients. Int. J. Mol. Sci. 2018, 19, 1176. [Google Scholar] [CrossRef] [PubMed]
- Dirkx, A.E.; Oude Egbrink, M.G.; Wagstaff, J.; Griffioen, A.W. Monocyte/macrophage infiltration in tumors: Modulators of angiogenesis. J. Leukoc. Biol. 2006, 80, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Longatto-Filho, A.; Martins, S.F. Gastric Cancer and Angiogenesis: Is VEGF a Useful Biomarker to Assess Progression and Remission? J. Gastric Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spessotto, P.; Fornasarig, M.; Pivetta, E.; Maiero, S.; Magris, R.; Mongiat, M.; Canzonieri, V.; De Paoli, P.; De Paoli, A.; Buonadonna, A.; et al. Probe-based confocal laser endomicroscopy for in vivo evaluation of the tumor vasculature in gastric and rectal carcinomas. Sci. Rep. 2017, 7, 9819–10963. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhao, L.; Dai, S.; Li, L.; Wang, F.; Shan, B. CCL5 secreted by tumor associated macrophages may be a new target in treatment of gastric cancer. Biomed. Pharmacother. 2016, 77, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, K.S.; Park, Y.S.; Kang, Y.H.; Lee, Y.J.; Lee, K.R.; Kim, H.K.; Ryu, K.W.; Bae, J.M.; Kim, S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: Possible role of a metastasis predictor. Eur. J. Cancer 2003, 39, 184–191. [Google Scholar] [CrossRef]
- Wang, T.; Wei, Y.; Tian, L.; Song, H.; Ma, Y.; Yao, Q.; Feng, M.; Wang, Y.; Gao, M.; Xue, Y. C-C motif chemokine ligand 5 (CCL5) levels in gastric cancer patient sera predict occult peritoneal metastasis and a poorer prognosis. Int. J. Surg. 2016, 32, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xu, X.; Luo, X.; Li, L.; Huang, B.; Li, X.; Tao, D.; Hu, J.; Gong, J. Role of RANTES and its receptor in gastric cancer metastasis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Hapfelmeier, A.; Blank, S.; Reiche, M.; Slotta-Huspenina, J.; Jesinghaus, M.; Novotny, A.; Schmidt, T.; Grosser, B.; Kohlruss, M.; et al. A novel pretherapeutic gene expression based risk score for treatment guidance in gastric cancer. Ann. Oncol. 2017, 29, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Taguri, M.; Imamura, H.; Sugimoto, N.; Nishikawa, K.; Yoshida, K.; Tan, P.; Tsuburaya, A. Genomic predictors of chemotherapy efficacy in advanced or recurrent gastric cancer in the GC0301/TOP002 phase III clinical trial. Cancer Lett. 2018, 412, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Fukui, R.; Nishimori, H.; Hata, F.; Yasoshima, T.; Ohno, K.; Nomura, H.; Yanai, Y.; Tanaka, H.; Kamiguchi, K.; Denno, R.; et al. Metastases-related genes in the classification of liver and peritoneal metastasis in human gastric cancer. J. Surg. Res. 2005, 129, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Furuhata, T.; Kimura, Y.; Kawakami, M.; Yamaguchi, K.; Tsuruma, T.; Zembutsu, H.; Hirata, K. The interplay between gastric cancer cell lines and PBMCs mediated by the CC chemokine RANTES plays an important role in tumor progression. J. Exp. Clin. Cancer Res. 2005, 24, 439–446. [Google Scholar] [PubMed]
- Sugasawa, H.; Ichikura, T.; Kinoshita, M.; Ono, S.; Majima, T.; Tsujimoto, H.; Chochi, K.; Hiroi, S.; Takayama, E.; Saitoh, D.; et al. Gastric cancer cells exploit CD4+ cell-derived CCL5 for their growth and prevention of CD8+ cell-involved tumor elimination. Int. J. Cancer 2008, 122, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bruno, A.; Gallo, C.; Pajardi, G.; Noonan, D.M.; Dallaglio, K. Cancer stem cells and the tumor microenvironment: Interplay in tumor heterogeneity. Connect. Tissue Res. 2015, 56, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.K.; Vanderbilt, D.B.; Salkeni, M.A.; Ruppert, J.M. Kruppel-like Pluripotency Factors as Modulators of Cancer Cell Therapeutic Responses. Cancer Res. 2016, 76, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, M.; Yang, X.; Zhang, X.; Zhang, Z.; Sun, Y.; Xu, B.; Hua, J.; He, Z.; Song, Z. Down-regulation of KLF5 in cancer-associated fibroblasts inhibit gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biol. Ther. 2017, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Graziosi, L.; Renga, B.; Cipriani, S.; D’Amore, C.; Francisci, D.; Bruno, A.; Baldelli, F.; Donini, A.; Fiorucci, S. CCR5 Antagonism by Maraviroc Reduces the Potential for Gastric Cancer Cell Dissemination. Transl. Oncol. 2013, 6, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Bria, E. Interfering with CCL5/CCR5 at the Tumor-Stroma Interface. Cancer Cell 2016, 29, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Chen, C.W.; Yang, C.L.; Tsai, I.M.; Hou, Y.C.; Chen, C.J.; Shan, Y.S. Tumor-Associated Macrophages Promote Epigenetic Silencing of Gelsolin through DNA Methyltransferase 1 in Gastric Cancer Cells. Cancer Immunol. Res. 2017, 5, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mutze, K.; Langer, R.; Schumacher, F.; Becker, K.; Ott, K.; Novotny, A.; Hapfelmeier, A.; Hofler, H.; Keller, G. DNA methyltransferase 1 as a predictive biomarker and potential therapeutic target for chemotherapy in gastric cancer. Eur. J. Cancer 2011, 47, 1817–1825. [Google Scholar] [CrossRef] [PubMed]

| Compound | Mechanism/Molecule | Cancer-Related Studies | References |
|---|---|---|---|
| Maraviroc Selzentry, Celsentri, UK-427857 (Pfizer) Approved by US FDA in 2007 for the treatment of HIV patients. | CCR5 antagonist | Enhanced cell killing mediated by DNA-damaging chemotherapeutic agents in breast cancer. | [54] |
| Reprogrammed immunosuppressive myeloid cells and reinvigorated antitumor immunity. | [32] | ||
| Repolarized TAMs. Objective clinical responses in advanced colorectal cancer patients with liver metastases (Phase I trial). | [53] | ||
| Decreased migration of CCR5+ regulatory T cells, reduced breast cancer growth in the lungs. | [55] | ||
| Vicriviroc SCH 417690, SCH-D(Merck) | Pyrimidine CCR5 entry inhibitor of HIV-1 | Enhanced cell killing mediated by DNA-damaging chemotherapeutic agents in breast cancer. | [54] |
| Inhibited invasiveness and metastatic potential in preclinical models of breast cancer. | [56] | ||
| TAK-779 (Takeda) | CCR5 antagonist, nonpeptide, quaternary ammonium derivative | Failed to protect from developing liver metastases in mice. | [57] |
| Reduced T-regs infiltration and tumor growth in a pancreatic cancer mouse model. | [58] | ||
| Met-CCL5 Met-RANTES | CCR5 inhibitor, competitive chemokine receptor blocker | Decreased mammary tumor cell invasion and activation of matrix metalloproteinases induced by mesenchymal stem cell-derived CCL9 and CCL5. | [59] |
| Decreased breast tumor growth, infiltrating macrophages, increased stromal development and necrosis in mice. | [60] | ||
| OTR4120 and OTR4131 | GAG mimetics, inhibit CCL5 binding to GAG | Strongly inhibited CCL5-induced migration and invasion of hepatocellular carcinoma. | [61] |
| Anibamine | CCR5 antagonist, natural product | Inhibited the proliferation of ovarian cancer cell lines, showing reduced cytotoxicity. | [62] |
| Inhibited prostate cancer cell growth, adhesion, and invasion. Reduced tumor growth in mice. | [63] | ||
| DT-13 | Steroidal saponin of dwarf lilyturf tuber | Inhibited gastric cancer cell migration by downregulation of both CCR5 and CCL5 expression. | [64] |
| Inhibited breast cancer cell proliferation, adhesion, and migration and lung metastasis in vivo by reducing VEGF, CCR5, HIF-1α. | [65] | ||
| Aplaviroc (GlaxoSmithKline) | CCR5 entry inhibitor | Developed for the treatment of HIV infection. Studies of Aplaviroc were discontinued because of liver toxicity. | [66] |
| GSK706769 (GlaxoSmithKline) | CCR5 antagonist | 2008 Completed phase I trial for HIV treatment. | https://adisinsight.springer.com/drugs/800023238 |
| INCB009471 (Incyte Corporation) | CCR5 inhibitor | Phase of Development: II (discontinued). HIV treatment. | https://aidsinfo.nih.gov/drugs/print/516/incb-9471/0/1/professional |
| Cenicriviroc TBR-652, TAK-652 (Takeda) | Inhibitor of CCR2 and CCR5 receptors | Completed study in a Phase IIb clinical trial for HIV treatment. | https://www.clinicaltrials.gov/ct2/show/NCT01338883 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldinucci, D.; Casagrande, N. Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer. Int. J. Mol. Sci. 2018, 19, 1477. https://doi.org/10.3390/ijms19051477
Aldinucci D, Casagrande N. Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer. International Journal of Molecular Sciences. 2018; 19(5):1477. https://doi.org/10.3390/ijms19051477
Chicago/Turabian StyleAldinucci, Donatella, and Naike Casagrande. 2018. "Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer" International Journal of Molecular Sciences 19, no. 5: 1477. https://doi.org/10.3390/ijms19051477




