Connexin 43-Based Therapeutics for Dermal Wound Healing
Abstract
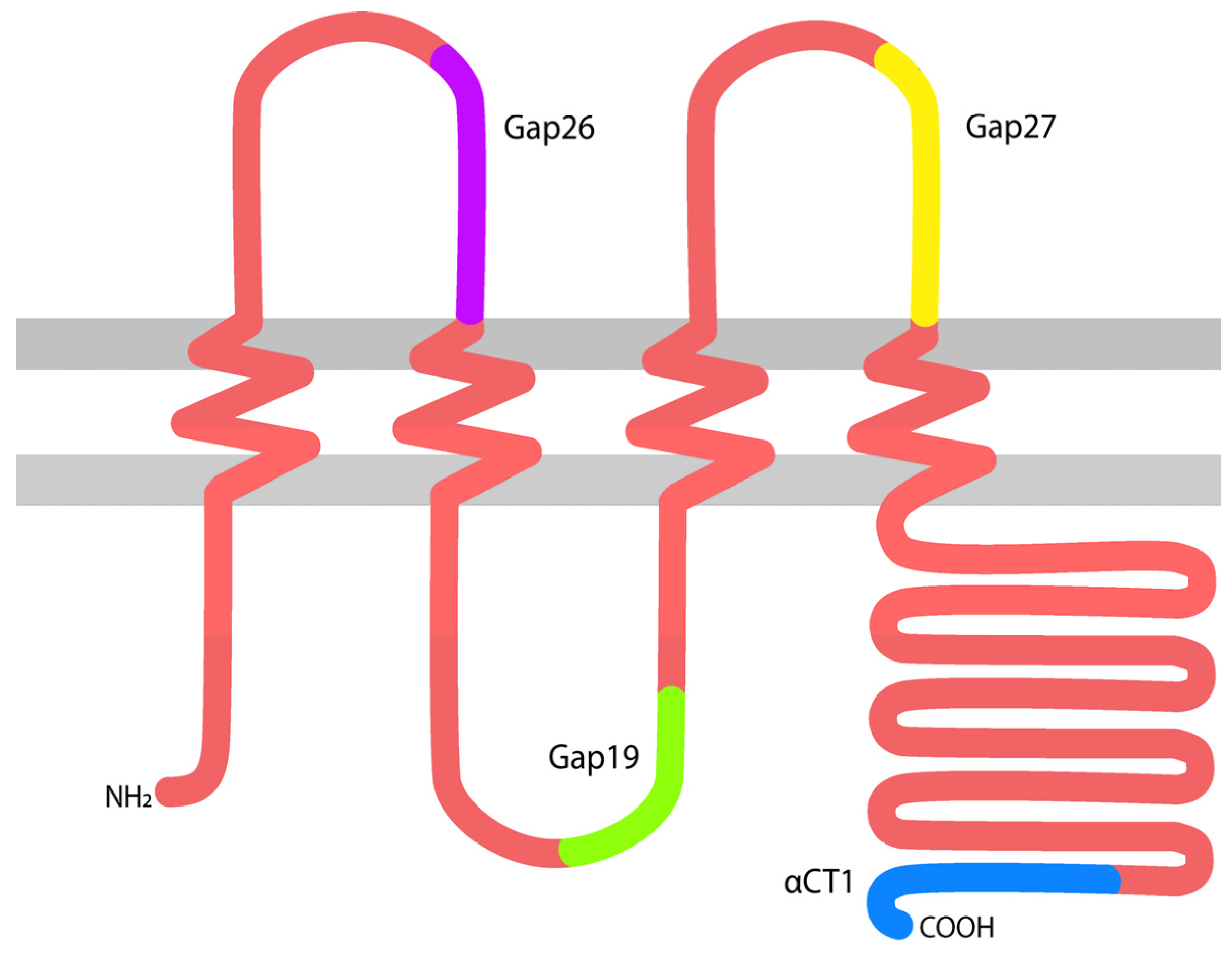
:1. Gap Junctions, Connexins, and Skin Wound Healing
2. Early Work on the Role of Cx43 in Cutaneous Wound Healing
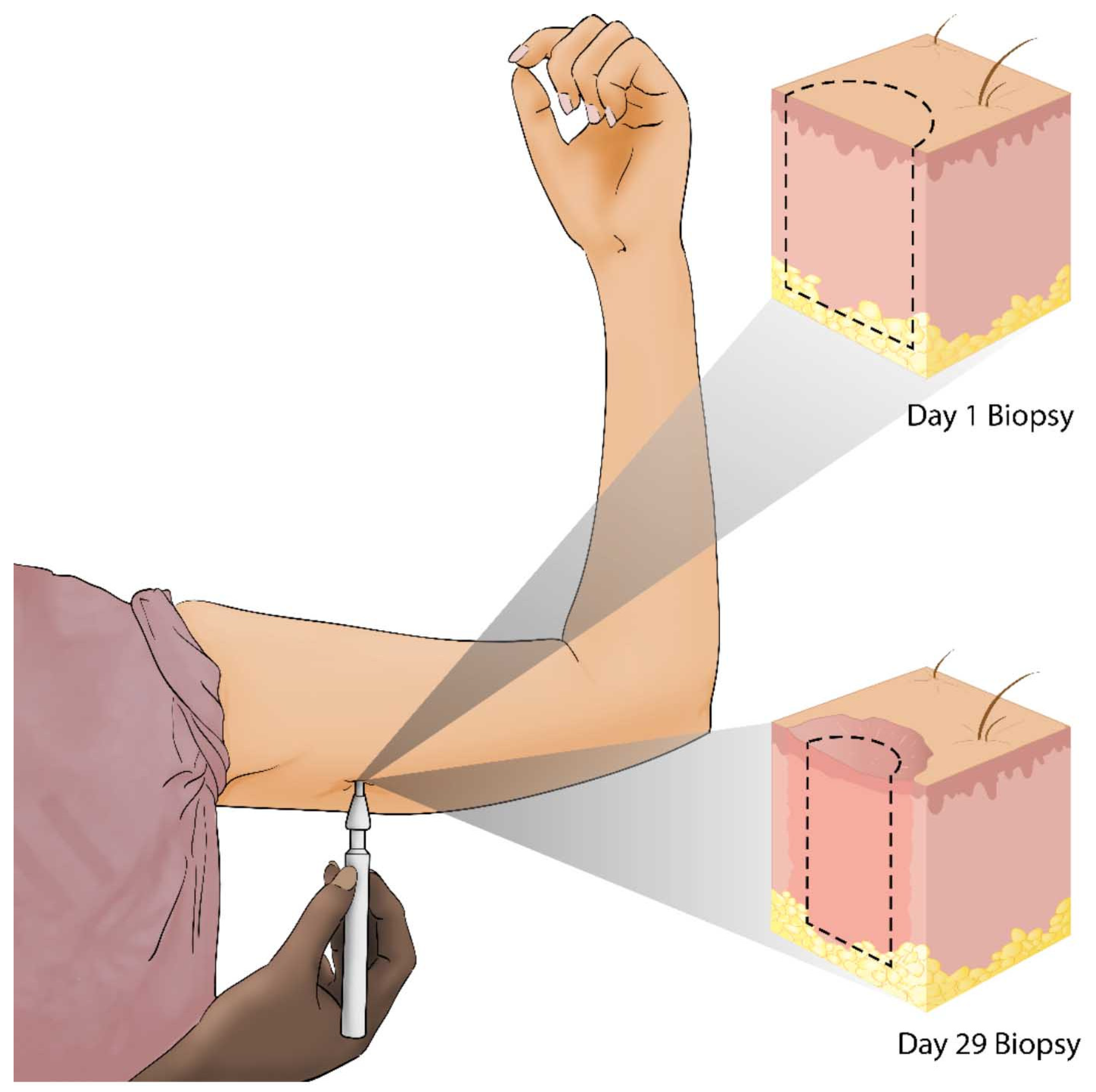
3. Preclinical Studies of αCT1 Peptide in Skin Wound Healing
4. Clinical Trials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Cx43 | Connexin 43 |
| αCT1 | Alpha connexin carboxyl terminus 1 |
| GJ | Gap Junction |
| ZO-1 | Zonula occludens-1 |
| CT | Carboxyl terminus |
References
- Sohl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalper, K.A.; Orellana, J.A.; Berthoud, V.M.; Sáez, J.C. Dysfunctions of the diffusional membrane pathways mediated by hemichannels in inherited and acquired human diseases. Curr. Vasc. Pharmacol. 2009, 7, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Sánchez, H.A.; Lee, S.C.; Altenberg, G.A.; Nathanson, M.H.; Sáez, J.C. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am. J. Physiol.-Cell Physiol. 2010, 299, C1504–C1515. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Wang, N.; Bol, M.; Decrock, E.; Ponsaerts, R.; Bultynck, G.; Dupont, G.; Leybaert, L. Connexin 43 hemichannels contribute to cytoplasmic Ca2+ oscillations by providing a bimodal Ca2+-dependent Ca2+ entry pathway. J. Biol. Chem. 2012, 287, 12250–12266. [Google Scholar] [CrossRef] [PubMed]
- Rhett, J.M.; Ghatnekar, G.S.; Palatinus, J.A.; O’Quinn, M.; Yost, M.J.; Gourdie, R.G. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008, 26, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ghatnekar, G.S.; Quinn, M.P.O.; Jourdan, L.J.; Gurjarpadhye, A.A.; Draughn, R.L.; Gourdie, R.G. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Future Med. 2009, 4, 205–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.E.M.; Easton, J.A.; Hodgins, M.B.; Wright, C.S. Connexins: Sensors of epidermal integrity that are therapeutic targets. FEBS Lett. 2014, 588, 1304–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churko, J.M.; Laird, D.W. Gap junction remodeling in skin repair following wounding and disease. Physiology 2013, 28, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Gourdie, R.G.; Ghatnekar, G.S.; O’Quinn, M.; Rhett, M.J.; Barker, R.J.; Zhu, C.; Jourdan, J.; Hunter, A.W. The unstoppable connexin43 carboxyl-terminus: New roles in gap junction organization and wound healing. Ann. N. Y. Acad. Sci. 2006, 1080, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Orgill, D.P.; Murphy, G.F. Chapter 4: The Pathophysiologic Basis for Wound Healing and Cutaneous Regeneration. In Biomaterials For Treating Skin Loss; Woodhead Publishing: Sawston, UK; Cambridge, UK; CRC Press: Boca Raton, FL, USA, 2009; pp. 25–57. ISBN 978-1-84569-363-3. [Google Scholar]
- Martin, P.; Lewis, J. Actin cables and epidermal movement in embryonic wound healing. Nature 1992, 360, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; D’Souza, D.; Martin, J.; Grose, R.; Cooper, L.; Maki, R.; McKercher, S.R. Wound healing in the PU. 1 null mouse—tissue repair is not dependent on inflammatory cells. Curr. Biol. 2003, 13, 1122–1128. [Google Scholar] [CrossRef]
- Martin, P. Mechanisms of wound healing in the embryo and fetus. Curr. Top. Dev. Biol. 1996, 32, 175–203. [Google Scholar] [PubMed]
- Ferguson, M.W.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004, 359, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Kimball, A.; Boniakowski, A.; Gallagher, K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diabetes Rep. 2018, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Berends, R.F.; Flint, D.J.; Martin, P.E.M. Cell motility in models of wounded human skin is improved by Gap27 despite raised glucose, insulin and IGFBP-5. Exp. Cell Res. 2013, 319, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Churko, J.M.; Kelly, J.J.; Macdonald, A.; Lee, J.; Sampson, J.; Bai, D.; Laird, D.W. The G60S Cx43 mutant enhances keratinocyte proliferation and differentiation. Exp. Dermatol. 2012, 21, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Rosado, L.; Singh, D.; Rincón-Arano, H.; Solan, J.L.; Lampe, P.D. CASK (LIN2) interacts with Cx43 in wounded skin and their coexpression affects cell migration. J. Cell Sci. 2012, 125, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, C.; Coutinho, P.; Frank, S.; Franke, S.; Law, L.; Martin, P.; Green, C.R.; Becker, D.L. Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 2003, 13, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.L.; Thrasivoulou, C.; Phillips, A.R.J. Connexins in wound healing; perspectives in diabetic patients. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Lorraine, C.; Wright, C.S.; Martin, P.E.M. Connexin43 plays diverse roles in co-ordinating cell migration and wound closure events. Biochem. Soc. Trans. 2015, 43, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, B.; Vinken, M.; Silva, T.C.; Araújo, C.M.M.; Aloia, T.P.A.; Chaible, L.M.; Mori, C.M.C.; Dagli, M.L.Z. Connexin 43 deficiency accelerates skin wound healing and extracellular matrix remodeling in mice. J. Dermatol. Sci. 2015, 79, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarzemany, R.; Jiang, G.; Larjava, H.; Häkkinen, L. Expression and function of connexin 43 in human gingival wound healing and fibroblasts. PLoS ONE 2015, 10, e0115524. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.A.; Tattersall, D.; O’Toole, E.A.; Kelsell, D.P. Connexins in epidermal homeostasis and skin disease. Biochim. Biophys. Acta 2012, 1818, 1952–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goliger, J.A.; Paul, D.L. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol. Biol. Cell 1995, 6, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.; Qiu, C.; Frank, S.; Tamber, K.; Becker, D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol. Int. 2003, 27, 525–541. [Google Scholar] [CrossRef]
- Moyer, K.E.; Davis, A.; Saggers, G.C.; Mackay, D.R.; Ehrlich, H.P. Wound healing: The role of gap junctional communication in rat granulation tissue maturation. Exp. Mol. Pathol. 2002, 72, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.G.; Phillips, A.; Becker, D.L. Connexin dynamics in the privileged wound healing of the buccal mucosa. Wound Repair Regen. 2013, 21, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Tarzemany, R.; Jiang, G.; Jiang, J.X.; Larjava, H.; Häkkinen, L. Connexin 43 Hemichannels Regulate the Expression of Wound Healing-Associated Genes in Human Gingival Fibroblasts. Sci. Rep. 2017, 7, 14157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarzemany, R.; Jiang, G.; Jiang, J.X.; Gallant-Behm, C.; Wiebe, C.; Hart, D.A.; Larjava, H.; Häkkinen, L. Connexin 43 regulates the expression of wound healing-related genes in human gingival and skin fibroblasts. Exp. Cell Res. 2018, 367, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Power, K.T.; Wang, C.M.; Martin, P.; Becker, D.L. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J. Cell Sci. 2006, 119, 5193–5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faniku, C.; O’Shaughnessy, E.; Lorraine, C.; Johnstone, S.R.; Graham, A.; Greenhough, S.; Martin, P.E.M. The Connexin Mimetic Peptide Gap27 and Cx43-Knockdown Reveal Differential Roles for Connexin43 in Wound Closure Events in Skin Model Systems. Int. J. Mol. Sci. 2018, 19, 604. [Google Scholar] [CrossRef] [PubMed]
- Brandner, J.M.; Houdek, P.; Hüsing, B.; Kaiser, C.; Moll, I. Connexins 26, 30, and 43: Differences among spontaneous, chronic, and accelerated human wound healing. J. Investig. Dermatol. 2004, 122, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Naranjo, A.; Cormie, P.; Serrano, A.E.; Hu, R.; O’Neill, S.; Wang, C.M.; Thrasivoulou, C.; Power, K.T.; White, A.; Serena, T.; et al. Targeting Cx43 and N.-Cadherin, Which Are Abnormally Upregulated in Venous Leg Ulcers, Influences Migration, Adhesion and Activation of Rho GTPases. PLoS ONE 2012, 7, e37374. [Google Scholar] [CrossRef] [PubMed]
- Kanapathy, M.; Simpson, R.; Madden, L.; Thrasivoulou, C.; Mosahebi, A.; Becker, D.L.; Richards, T. Upregulation of epidermal gap junctional proteins in patients with venous disease. Br. J. Surg. 2018, 105, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ghatnekar, G.S.; Grek, C.L.; Armstrong, D.G.; Desai, S.C.; Gourdie, R.G. The effect of a connexin43-based Peptide on the healing of chronic venous leg ulcers: A multicenter, randomized trial. J. Investig. Dermatol. 2015, 135, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Baquerizo Nole, K.L.; Fox, J.D.; Liu, S.N. Healing refractory venous ulcers: New treatments offer hope. J. Investig. Dermatol. 2015, 135, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.L.; Montgomery, J.; Sharma, M.; Ravi, A.; Rajkumar, J.S.; Moyer, K.E.; Gourdie, R.G.; Ghatnekar, G.S. A Multicenter Randomized Controlled Trial Evaluating a Cx43-Mimetic Peptide in Cutaneous Scarring. J. Investig. Dermatol. 2017, 137, 620–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollok, S.; Pfeiffer, A.-C.; Lobmann, R.; Wright, C.S.; Moll, I.; Martin, P.E.M.; Brandner, J.M. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J. Cell. Mol. Med. 2011, 15, 861–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grek, C.L.; Prasad, G.M.; Viswanathan, V.; Armstrong, D.G.; Gourdie, R.G.; Ghatnekar, G.S. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair Regen. 2015, 23, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grek, C.L.; Rhett, J.M.; Ghatnekar, G.S. Cardiac to cancer: Connecting connexins to clinical opportunity. FEBS Lett. 2014, 588, 1349–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, A.W.; Barker, R.J.; Zhu, C.; Gourdie, R.G. Zonula Occludens-1 Alters Connexin43 Gap Junction Size and Organization by Influencing Channel Accretion. Mol. Biol. Cell 2005, 16, 5686–5698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhett, J.M.; Jourdan, J.; Gourdie, R.G. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell 2011, 22, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Palatinus, J.A.; He, H.; Iyyathuraia, J.; Jordan, J.; McGowan, F.X.; Schey, K.; Bultynck, G.; Zhang, Z.; Gourdie, R.G. Phosphorylation of Connexin43 at Serine368 is Necessary for Induction of Cardioprotection by a Connexin43 Carboxyl-Terminal Mimetic Peptide. Circulation 2016, 134, A16380. [Google Scholar]
- O’Quinn, M.P.; Palatinus, J.A.; Harris, B.S.; Hewett, K.W.; Gourdie, R.G. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ. Res. 2011, 108, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Ongstad, E.L.; O’Quinn, M.P.; Ghatnekar, G.S.; Yost, M.J.; Gourdie, R.G. A Connexin43 Mimetic Peptide Promotes Regenerative Healing and Improves Mechanical Properties in Skin and Heart. Adv. Wound Care 2013, 2, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanz, J.; Pusch, J.; Hansmann, J.; Walles, H. Vascularised human tissue models: A new approach for the refinement of biomedical research. J. Biotechnol. 2010, 148, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Bryant, Z.J.; Ghatnekar, G.; Singh, U.P.; Gourdie, R.G.; Potts, J.D. A synthetic connexin 43 mimetic peptide augments corneal wound healing. Exp. Eye Res. 2013, 115, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.; Ghatnekar, G.; Gourdie, R.G.; Potts, J.D. Impact of the Controlled Release of a Connexin 43 Peptide on Corneal Wound Closure in an STZ Model of Type I Diabetes. PLoS ONE 2014, 9, e86570. [Google Scholar] [CrossRef] [PubMed]
- Soder, B.L.; Propst, J.T.; Brooks, T.M.; Goodwin, R.L.; Friedman, H.I.; Yost, M.J.; Gourdie, R.G. The connexin43 carboxyl-terminal peptide ACT1 modulates the biological response to silicone implants. Plast. Reconstr. Surg. 2009, 123, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Koulakoff, A.; Giaume, C. Astroglial Connexins as a Therapeutic Target for Alzheimer’s Disease. Curr. Pharm. Des. 2017, 23, 4958–4968. [Google Scholar] [CrossRef] [PubMed]
- Willebrords, J.; Maes, M.; Crespo Yanguas, S.; Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 2017, 180, 144–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.L.; Phillips, A.R.; Duft, B.J.; Kim, Y.; Green, C.R. Translating connexin biology into therapeutics. Semin. Cell Dev. Biol. 2016, 50, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Iyyathurai, J.; D’hondt, C.; Wang, N.; De Bock, M.; Himpens, B.; Retamal, M.A.; Stehberg, J.; Leybaert, L.; Bultynck, G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: Gap junctions versus hemichannels. Neuropharmacology 2013, 75, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Obert, E.; Strauss, R.; Brandon, C.; Grek, C.L.; Ghatnekar, G.S.; Gourdie, R.G.; Rohrer, B. Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, αCT1, reduces VEGF-dependent RPE pathophysiology. J. Mol. Med. 2017, 95, 535–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naus, C.C.; Giaume, C. Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J. Transl. Med. 2016, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Lin, J.C.; Hung, C.Y.; Li, C.H.; Lin, S.F.; Yeh, H.I.; Huang, J.L.; Lo, C.P.; Haugan, K.; Larsen, B.D.; et al. Gap junction modifier rotigaptide decreases the susceptibility to ventricular arrhythmia by enhancing conduction velocity and suppressing discordant alternans during therapeutic hypothermia in isolated rabbit hearts. Heart Rhythm 2016, 13, 251–261. [Google Scholar] [CrossRef] [PubMed]


| Clinical Trial Phase | Phase I | Phase II | ||
|---|---|---|---|---|
| Wound Type | Healthy Human Dermal Wounds | Venous Leg Ulcers | Diabetic Foot Ulcers | Cutaneous Scarring/Laparoscopic Incisions |
| Treatment Regimen | Immediately after wounding and 24 h later | Twice during the 1st week and once a week thereafter | Twice during the 1st week and once a week thereafter | Immediately after wounding and 24 h later |
| Patients | 49 | 92 | 92 | 91 |
| No Adverse Effects | ✓ | ✓ | ✓ | ✓ |
| Mean Percent Ulcer Area Reduction at 12 Weeks | - | 79% αCT1 vs. 36% control | 94% αCT1 vs. 52% control | - |
| Incidence of Complete Ulcer Closure at 12 Weeks | - | 57% αCT1 vs. 28% control | 81% αCT1 vs. 50% control | - |
| Comparative Vancouver Scar Scale Scores at 9 Months | - | - | - | 47% better for αCT1 compared to within-patient controls |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montgomery, J.; Ghatnekar, G.S.; Grek, C.L.; Moyer, K.E.; Gourdie, R.G. Connexin 43-Based Therapeutics for Dermal Wound Healing. Int. J. Mol. Sci. 2018, 19, 1778. https://doi.org/10.3390/ijms19061778
Montgomery J, Ghatnekar GS, Grek CL, Moyer KE, Gourdie RG. Connexin 43-Based Therapeutics for Dermal Wound Healing. International Journal of Molecular Sciences. 2018; 19(6):1778. https://doi.org/10.3390/ijms19061778
Chicago/Turabian StyleMontgomery, Jade, Gautam S. Ghatnekar, Christina L. Grek, Kurtis E. Moyer, and Robert G. Gourdie. 2018. "Connexin 43-Based Therapeutics for Dermal Wound Healing" International Journal of Molecular Sciences 19, no. 6: 1778. https://doi.org/10.3390/ijms19061778





