Probing the Effect of Physiological Concentrations of IL-6 on Insulin Secretion by INS-1 832/3 Insulinoma Cells under Diabetic-Like Conditions
Abstract
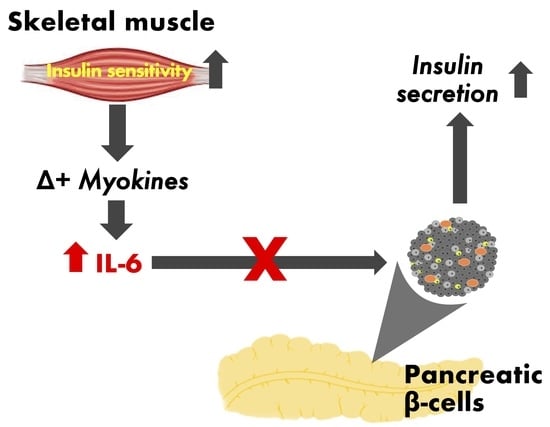
:1. Introduction
2. Results
2.1. Acute IL-6 Treatment Has No Effect on Glucose-Stimulated Insulin Secretion (GSIS) by INS-1 832/3 Cells at an Exercise-Relevant Concentration
2.2. Acute IL-6 Treatment Neither Worsens nor Improves Insulin Secretory Function by INS-1 832/3 Cells Exposed to Diabetic-Like Conditions
2.3. Contraction-Mediated Increases in IL-6 from C2C12 Myotubes Has No Effect on Insulin Secretion by INS-1 832/3 Cells
3. Discussion
3.1. IL-6 Effects on INS-1 832/3 Cells Exposed to Diabetic-Like Conditions
3.2. Exercise-Induced IL-6 Effects against IL-1β-Induced INS-1 832/3 Cell Dysfunction
4. Materials and Methods
4.1. INS-1E 832/3 Cell Culture
4.2. Insulin Secretion
4.3. Muscle-Conditioned Medium
4.4. Glucose Uptake
4.5. IL-6 Secretion
4.6. Cell Density
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| β-cell | Pancreatic beta-cell |
| T2D | Type 2 diabetes |
| TD1 | Type 1 diabetes |
| GSIS | Glucose-stimulated insulin secretion |
| NEFA | Non-esterified fatty acids |
| KRH | Krebs-Ringer HEPES buffer |
| CM | Conditioned medium |
| BSA | Bovine serum albumin |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| DAPI | 4′,6-diamidino-2′-phenylindole dihydrochloride |
| EPS | Electrical pulse stimulation |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
References
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.S. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C.; White, R.D. Physical activity/exercise and type 2 diabetes. Diabetes Care 2006, 29, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Exercise-induced increase in muscle insulin sensitivity. J. Appl. Physiol. 2005, 99, 338–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dela, F.; von Linstow, M.E.; Mikines, K.J.; Galbo, H. Physical training may enhance β-cell function in type 2 diabetes. Am. J. Physiol. 2004, 287, E1024–E1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, T.P.J.; Haus, J.M.; Kelly, K.R.; Rocco, M.; Kashyap, S.R.; Kirwan, J.P. Improved pancreatic β-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010, 33, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-E.; Choi, K.-M.; Yoon, I.-H.; Shin, J.Y.; Kim, J.S.; Park, W.Y.; Han, D.J.; Kim, S.C.; Ahn, C.; Kim, J.Y.; et al. IL-6 protects pancreatic islet β cells from pro-inflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transpl. Immunol. 2004, 13, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pold, R.; Jensen, L.S.; Jessen, N.; Buhl, E.S.; Schmitz, O.; Flyvbjerg, A.; Fujii, N.; Goodyear, L.J.; Gotfredsen, C.F.; Brand, C.L.; et al. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 2005, 54, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Bouzakri, K.; Plomgaard, P.; Berney, T.; Donath, M.Y.; Pedersen, B.K.; Halban, P.A. Bimodal effect on pancreatic β-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes 2011, 60, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.P.; Solomon, T.P.J. Do skeletal muscle-secreted factors influence the function of pancreatic β-cells? Am. J. Physiol. 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Eckardt, K.; Holven, K.B.; Jensen, J.; Eckel, J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE 2013, 8, E62008–E62012. [Google Scholar] [CrossRef] [PubMed]
- Paula, F.M.M.; Leite, N.C.; Vanzela, E.C.; Kurauti, M.A.; Freitas-Dias, R.; Carneiro, E.M.; Boschero, A.C.; Zoppi, C.C. Exercise increases pancreatic β-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J. 2015, 29, 1805–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paula, F.M.M.; Leite, N.C.; Borck, P.C.; Freitas-Dias, R.; Cnop, M.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R.; Marchetti, P.; Boschero, A.C.; Zoppi, C.C.; et al. Exercise training protects human and rodent β cells against endoplasmic reticulum stress and apoptosis. FASEB J. 2018, 32, 1524–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and α cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Southern, C.; Schulster, D.; Green, I.C. Inhibition of insulin-secretion from rat islets of langerhans by interleukin-6—An effect distinct from that of interleukin-1. Biochem. J. 1990, 272, 243–245. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.M.; Lu, C.; Corbin, K.L.; Sharma, P.R.; Dula, S.B.; Carter, J.D.; Ramadan, J.W.; Xin, W.; Lee, J.K.; Nunemaker, C.S. Circulating levels of IL-1B + IL-6 cause ER stress and dysfunction in islets from prediabetic male mice. Endocrinology 2013, 154, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Krause, M.; Bittencourt, A.; Homem de Bittencourt, P.I.; McClenaghan, N.H.; Flatt, P.R.; Murphy, C.; Newsholme, P. Physiological concentrations of interleukin-6 directly promote insulin secretion, signal transduction, nitric oxide release, and redox status in a clonal pancreatic β-cell line and mouse islets. J. Endocrinol. 2012, 214, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Imai, J.; Yamada, T.; Ishigaki, Y.; Kaneko, K.; Uno, K.; Hasegawa, Y.; Ishihara, H.; Oka, Y.; Katagiri, H. Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic β-cells: Potential involvement of the PLC-IP3-dependent pathway. Diabetes 2011, 60, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Lee, Y.-J.; Park, E.Y.; Jun, H.-S. Interleukin-6 treatment induces β-cell apoptosis via STAT-3-mediated nitric oxide production. Diabetes Metab. Res. Rev. 2011, 27, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.S.; Christensen, D.P.; Lundh, M.; Dahllöf, M.S.; Haase, T.N.; Velasquez, J.M.; Laye, M.J.; Mandrup-Poulsen, T.; Solomon, T.P. Skeletal muscle to pancreatic β-cell cross-talk: The effect of humoral mediators liberated by muscle contraction and acute exercise on β-cell apoptosis. J. Clin. Endocrinol. Metab. 2015, 100, E1289–E1298. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, Y.; Yang, J. Cytokines in the progression of pancreatic β-cell dysfunction. Int. J. Endocrinol. 2010, 2010, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Welters, H.J.; Tadayyon, M.; Scarpello, J.H.B.; Smith, S.A.; Morgan, N.G. Mono-unsaturated fatty acids protect against β-cell apoptosis induced by saturated fatty acids, serum withdrawal or cytokine exposure. FEBS Lett. 2004, 560, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Li, J.; Shen, M.; Jia, W.; Chen, N.; Chen, T.; Su, D.; Tian, H.; Zheng, S.; Dai, Y.; et al. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic β-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes 2010, 59, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Keller, P.; Keller, C.; Fischer, C.; Hiscock, N.; van Hall, G.; Plomgaard, P.; Febbraio, M.A. Muscle-derived interleukin-6: Lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. Eur. J. Physiol. 2003, 446, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Ploug, T.; Galbo, H. Increased muscle glucose uptake after exercise: No need for insulin during exercise. Diabetes 1985, 34, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, J.; Affourtit, C. Novel insights into pancreatic β-cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells. Biochem. J. 2013, 456, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Cui, Q.; Yang, B.; Hou, Y.; Wang, H.; Xu, Y.; Wang, D.; Zhang, Q.; Pi, J. The impairment of glucose-stimulated insulin secretion in pancreatic β-cells caused by prolonged glucotoxicity and lipotoxicity is associated with elevated adaptive antioxidant response. Food Chem. Toxicol. 2017, 100, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Schumann, D.M.; Faulenbach, M.; Ellingsgaard, H.; Perren, A.; Ehses, J.A. Islet inflammation in type 2 diabetes: From metabolic stress to therapy. Diabetes Care 2008, 31 (Suppl. 2), S161–S164. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Sandler, S.; Welsh, N.; Cetkovic-Cvrlje, M.; Nieman, A.; Geller, D.A.; Pipeleers, D.G.; Bendtzen, K.; Hellerström, C. Cytokines suppress human islet function irrespective of their effects on nitric-oxide generation. J. Clin. Investig. 1994, 93, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, I.; Ladrière, L.; Cardozo, A.K.; Dogusan, Z.; Cnop, M.; Eizirik, D.L. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κb and endoplasmic reticulum stress. Endocrinology 2004, 145, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.J.; Stadler, K.; Lu, D.; Gleason, E.; Han, A.; Donohoe, D.R.; Rogers, R.C.; Hermann, G.E.; Karlstad, M.D.; Collier, J.J. IL-1β reciprocally regulates chemokine and insulin secretion in pancreatic β-cells via NF-κB. Am. J. Physiol. 2015, 309, E715–E726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, J.J.; Burke, S.J.; Eisenhauer, M.E.; Lu, D.; Sapp, R.C.; Frydman, C.J.; Campagna, S.R. Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS ONE 2011, 6, E22485. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J.S. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Mougios, V.; Kouidi, E.; Kyparos, A.; Deligiannis, A. Effect of exercise on the proportion of unsaturated fatty acids in serum of untrained middle aged individuals. Br. J. Sports Med. 1998, 32, 58–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose-John, S. IL-6 Trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Roytblat, L.; Rachinsky, M.; Fisher, A.; Greemberg, L.; Shapira, Y.; Douvdevani, A.; Gelman, S. Raised Interleukin-6 levels in obese patients. Obesity 2000, 8, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Klover, P.J.; Zimmers, T.A.; Koniaris, L.G.; Mooney, R.A. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 2003, 52, 2784–2789. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.H.; Kampf, J.P.; Kwan, T.; Zhu, B.; Kleinfeld, A.M. Fatty acid-specific fluorescent probes and their use in resolving mixtures of unbound free fatty acids in equilibrium with albumin. Biochemistry 2006, 45, 14263–14274. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ueda-Wakagi, M.; Sato, T.; Kawasaki, K.; Sawada, K.; Kawabata, K.; Ashida, H. Measurement of glucose uptake in cultured cells. Curr. Protoc. Pharmacol. 2015, 71, 12.14.1–12.12.26. [Google Scholar]
- Barlow, J.; Jensen, V.H.; Affourtit, C. Uncoupling protein-2 attenuates palmitoleate protection against the cytotoxic production of mitochondrial reactive oxygen species in INS-1E insulinoma cells. Redox Biol. 2015, 4, 14–22. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barlow, J.; Carter, S.; Solomon, T.P.J. Probing the Effect of Physiological Concentrations of IL-6 on Insulin Secretion by INS-1 832/3 Insulinoma Cells under Diabetic-Like Conditions. Int. J. Mol. Sci. 2018, 19, 1924. https://doi.org/10.3390/ijms19071924
Barlow J, Carter S, Solomon TPJ. Probing the Effect of Physiological Concentrations of IL-6 on Insulin Secretion by INS-1 832/3 Insulinoma Cells under Diabetic-Like Conditions. International Journal of Molecular Sciences. 2018; 19(7):1924. https://doi.org/10.3390/ijms19071924
Chicago/Turabian StyleBarlow, Jonathan, Steven Carter, and Thomas P. J. Solomon. 2018. "Probing the Effect of Physiological Concentrations of IL-6 on Insulin Secretion by INS-1 832/3 Insulinoma Cells under Diabetic-Like Conditions" International Journal of Molecular Sciences 19, no. 7: 1924. https://doi.org/10.3390/ijms19071924