Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study
Abstract
:1. Introduction
2. Results
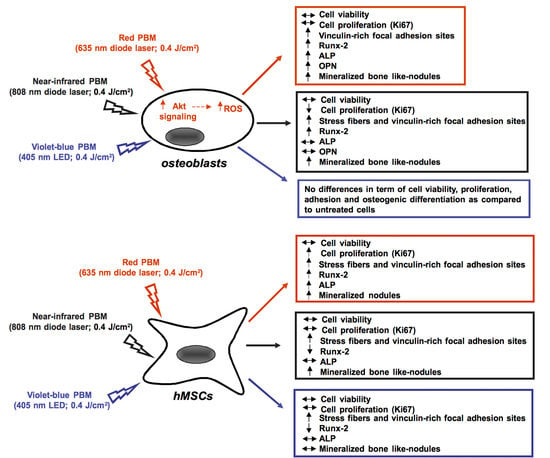
2.1. Human Osteoblast Viability, Proliferation, Adhesion and Differentiation
2.2. Human MSC Viability, Proliferation, Adhesion and Osteogenic Differentiation
2.3. Osteoblast Responses to PBM with 635 nm are Mediated by Akt Signaling Activation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. PBM Treatments
4.3. Cell Viability
4.4. Morphological Analyses
4.4.1. Phase Contrast Microscopy
4.4.2. Confocal Laser Scanning Microscopy
4.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.6. Fluorescent Mineralized Nodules Assay
4.7. Western Blotting
4.8. ROS Generation Detection
4.9. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Grover, V.; Bhaskar, N.; Kaur, R.K.; Jain, A. Periodontal Infectogenomics. Inflamm. Regen. 2018, 38, 8. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, D.; Zhang, Y.; Li, M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem. Cell Biol. 2018, 149, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care (New Rochelle) 2013, 2, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tevlin, R.; Walmsley, G.G.; Marecic, O.; Hu, M.S.; Wan, D.C.; Longaker, M.T. Stem and progenitor cells: Advancing bone tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Pearlin Nayak, S.; Manivasagam, G.; Sen, D. Progress of Regenerative Therapy in Orthopedics. Curr. Osteoporos. Rep. 2018, 16, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Benayahu, D.; Maltz, L.; Oron, U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed. Laser Surg. 2005, 23, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Chellini, F.; Sassoli, C.; Francini, F.; Pini, A.; Squecco, R.; Nosi, D.; Bani, D.; Zecchi-Orlandini, S.; Formigli, L. Photoactivation of bone marrow mesenchymal stromal cells with diode laser: Effects and mechanisms of action. J. Cell. Physiol. 2013, 228, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Ceccarelli, G.; Minzioni, P.; Vercellino, M.; Benedetti, L.; De Angelis, M.G.; Imbriani, M.; Visai, L. Investigation of low-level laser therapy potentiality on proliferation and differentiation of human osteoblast-like cells in the absence/presence of osteogenic factors. J. Biomed. Opt. 2013, 18, 128006. [Google Scholar] [CrossRef] [PubMed]
- Amid, R.; Kadkhodazadeh, M.; Ahsaie, M.G.; Hakakzadeh, A. Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. Lasers Med. Sci. 2014, 5, 163–170. [Google Scholar]
- Migliario, M.; Pittarella, P.; Fanuli, M.; Rizzi, M.; Renò, F. Laser-induced osteoblast proliferation is mediated by ROS production. Lasers Med. Sci. 2014, 29, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Matos, A.A.; Santesso, M.R.; Tokuhara, C.K.; Leite, A.L.; Bagnato, V.S.; Machado, M.A.; Peres-Buzalaf, C.; Oliveira, R.C. Low intensity lasers differently induce primary human osteoblast proliferation and differentiation. J. Photochem. Photobiol. B 2016, 163, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Asefi, S.; Allahdadi, M.; Kalhori, K.A. Effect of Photobiomodulation on Mesenchymal Stem Cells. Photomed. Laser Surg. 2016, 34, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Diniz, I.M.A.; Carreira, A.C.O.; Sipert, C.R.; Uehara, C.M.; Moreira, M.S.N.; Freire, L.; Pelissari, C.; Kossugue, P.M.; de Araújo, D.R.; Sogayar, M.C.; et al. Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhBMP4-loaded hydrogel directs hard tissue bioengineering. J. Cell. Physiol. 2017, 233, 4907–4918. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Virdi, A.; Rezaei, F.; Chien, S. Comparison of the in vitro effects of low-level laser therapy and low-intensity pulsed ultrasound therapy on bony cells and stem cells. Prog. Biophys. Mol. Biol. 2018, 133, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Deana, A.M.; de Souza, A.M.; Teixeira, V.P.; Mesquita-Ferrari, R.A.; Bussadori, S.K.; Fernandes, K.P.S. The impact of photobiomodulation on osteoblast-like cell: A review. Lasers Med. Sci. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poppi, R.R.; Da Silva, A.L.; Nacer, R.S.; Vieira, R.P.; de Oliveira, L.V.F.; de Faria Júnior, N.S.; Carvalho, P.D.T.C. Evaluation of the osteogenic effect of low-level laser therapy (808 nm and 660 nm) on bone defects induced in the femurs of female rats submitted to ovariectomy. Lasers Med. Sci. 2011, 26, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.R.; Ribeiro, D.A.; Rodrigues, N.C.; Tim, C.; Santos, A.A.; Parizotto, N.A.; de Araujo, H.S.; Driusso, P.; Rennó, A.C. Effects of low-level laser therapy on the expression of osteogenic genes related in the initial stages of bone defects in rats. J. Biomed. Opt. 2013, 18, 038002. [Google Scholar] [CrossRef] [PubMed]
- Tim, C.R.; Pinto, K.N.; Rossi, B.R.; Fernandes, K.; Matsumoto, M.A.; Parizotto, N.A.; Rennó, A.C. Low-level laser therapy enhances the expression of osteogenic factors during bone repair in rats. Lasers Med. Sci. 2014, 29, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Medalha, C.C.; Santos, A.L.; Veronez Sde, O.; Fernandes, K.R.; Magri, A.M.; Renno, A.C. Low level laser therapy accelerates bone healing in spinal cord injured rats. J. Photochem. Photobiol. B 2016, 159, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Santinoni, C.D.; Oliveira, H.F.; Batista, V.E.; Lemos, C.A.; Verri, F.R. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: A systematic review. J. Photochem. Photobiol. B 2017, 169, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.F.; da Graças Vilela Goulart, M.; Giannasi, L.C.; Hiraoka, C.M.; de Fátima Santana Melo, G.; de Sousa, A.G.V.; Nóbrega, C.J.P.; Zangaro, R.A.; Salgado, M.A.C. Effects of the GaAlAs diode laser (780 nm) on the periodontal tissues during orthodontic tooth movement in diabetes rats: Histomorphological and immunohistochemical analysis. Lasers Med. Sci. 2017, 32, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Noba, C.; Mello-Moura, A.C.V.; Gimenez, T.; Tedesco, T.K.; Moura-Netto, C. Laser for bone healing after oral surgery: Systematic review. Lasers Med. Sci. 2018, 33, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Renno, A.C.; McDonnell, P.A.; Parizotto, N.A.; Laakso, E.L. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed. Laser Surg. 2007, 25, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Bölükbaşı Ateş, G.; Ak Can, A.; Gülsoy, M. Investigation of photobiomodulation potentiality by 635 and 809 nm lasers on human osteoblasts. Lasers Med. Sci. 2017, 32, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose-Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Squecco, R.; Tani, A.; Idrizaj, E.; Nosi, D.; Giannelli, M.; Zecchi-Orlandini, S. Low intensity 635 nm diode laser irradiation inhibits fibroblast-myofibroblast transition reducing TRPC1 channel expression/activity: New perspectives for tissue fibrosis treatment. Lasers Surg. Med. 2016, 48, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: In vitro study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. The effects of diode laser on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide adherent to titanium oxide surface of dental implants. An in vitro study. Lasers Med. Sci. 2016, 31, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J. Biomed. Opt. 2014, 19, 105001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujihara, S.; Yokozeki, M.; Oba, Y.; Higashibata, Y.; Nomura, S.; Moriyama, K. Function and regulation of osteopontin in response to mechanical stress. J. Bone Miner. Res. 2006, 21, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, W.H. Role of vinculin in cellular mechanotransduction. Cell Biol. Int. 2016, 40, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.F.; Zhang, H.; Yuan, X.; Li, J.; Wei, Y.J.; Hu, S.S. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: Proliferation, growth factors secretion and myogenic differentiation. Lasers Surg. Med. 2008, 40, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, W.; Wu, Y.; Hou, J.; Nie, Y.; Gu, H.; Li, J.; Hu, S.; Zhang, H. MicroRNA-193 pro-proliferation effects for bone mesenchymal stem cells after low-level laser irradiation treatment through inhibitor of growth family, member 5. Stem Cells Dev. 2012, 21, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Wang, J.; Gong, D.X.; Gu, H.Y.; Hu, S.S.; Zhang, H. Effects of low-level laser irradiation on mesenchymal stem cell proliferation: A microarray analysis. Lasers Med. Sci. 2012, 27, 509–519. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Hwang, H.S.; Shim, J.H. Comparison of adult stem cells derived from multiple stem cell niches. Biotechnol. Lett. 2016, 38, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Wada, H.; Kimura, Y.; Ueda, K.; Kioka, N. Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes. J. Cell Sci. 2017, 130, 989–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Mosca, C.; Bosetti, M.; Cannas, M. The role of mechanical stretching in the activation and localization of adhesion proteins and related intracellular molecules. J. Cell. Biochem. 2011, 112, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Yashiro, M.; Nishioka, N.; Hirakawa, K.; Olden, K.; Roberts, J.D. PI3K/Akt signalling is required for the attachment and spreading, and growth in vivo of metastatic scirrhous gastric carcinoma. Br. J. Cancer 2012, 106, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Rubashkin, M.G.; Cassereau, L.; Bainer, R.; DuFort, C.C.; Yui, Y.; Ou, G.; Paszek, M.J.; Davidson, M.W.; Chen, Y.Y.; Weaver, V.M. Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer Res. 2014, 74, 4597–4611. [Google Scholar] [CrossRef] [PubMed]
- Le, O.T.; Cho, O.Y.; Tran, M.H.; Kim, J.A.; Chang, S.; Jou, I.; Lee, S.Y. Phosphorylation of phosphatidylinositol 4-phosphate 5-kinase γ by Akt regulates its interaction with talin and focal adhesion dynamics. Biochim. Biophys. Acta 2015, 1853, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Bellosta, P.; Grassi, R.; Basilico, C.; Mansukhani, A. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J. Cell. Physiol. 2008, 215, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Signaling pathways affecting skeletal health. Curr. Osteoporos. Rep. 2012, 10, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Pramojanee, S.N.; Phimphilai, M.; Chattipakorn, N.; Chattipakorn, S.C. Possible roles of insulin signaling in osteoblasts. Endocr. Res. 2014, 39, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hung, H.S.; Hsu, S.H. Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibly via PI3K signal pathway. Lasers Surg. Med. 2008, 40, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xing, D.; Gao, X.; Wu, S. Low-power laser irradiation promotes cell proliferation by activating PI3K/Akt pathway. J. Cell. Physiol. 2009, 219, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xing, D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J. Biomed. Sci. 2009, 16, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Tang, Y.; Xing, D. Activation of nuclear estrogen receptors induced by low-power laser irradiation via PI3-K/Akt signaling cascade. J. Cell. Physiol. 2013, 228, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Machado, J.A.; Lima e Silva, F.C.; Cunha, E.P.; Calsolari, M.R.; Costa, D.C.; Perilo, C.S.; Horta, B.C.; Ferreira, I.C.; Chaves, M.M. Modulation of the production of reactive oxygen species (ROS) by cAMP-elevating agents in granulocytes from diabetic patients: An Akt/PKB-dependent phenomenon. Diabetes Metab. 2006, 32, 331–335. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Tu, Y.; Abu-Yousif, A.O.; Hasan, T. Photodynamic activation as a molecular switch to promote osteoblast cell differentiation via AP-1 activation. Sci. Rep. 2015, 5, 13114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.W.; Ding, K.; Dai, X.Y.; Ling, W.Q. β-aminoisobutyric acid accelerates the proliferation and differentiation of MC3T3-E1 cells via moderate activation of ROS signaling. J. Chin. Med. Assoc. 2018, 81, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Shibata, Y.; Pugdee, K.; Abiko, Y.; Ogata, Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 2007, 59, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ayala, V.; Santillán, G.; Boland, R. Activation of the PI3K/Akt signaling pathway through P2Y2 receptors by extracellular ATP is involved in osteoblastic cell proliferation. Arch. Biochem. Biophys. 2011, 513, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Peña, V.B.; Scolaro, L.A.; Santillán, G.E. ATP and UTP stimulate bone morphogenetic protein-2,-4 and -5 gene expression and mineralization by rat primary osteoblasts involving PI3K/AKT pathway. Exp. Cell Res. 2013, 319, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.W.; Pfau, B.; Jones, D.; Meyer, T. Stimulation of primary osteoblasts with ATP induces transient vinculin clustering at sites of high intracellular traction force. J. Mol. Histol. 2014, 45, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Schakman, O.; Louis, P.; Ruegg, U.T.; Dietrich, A.; Birnbaumer, L.; Gailly, P. Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. J. Biol. Chem. 2012, 287, 14524–14534. [Google Scholar] [CrossRef] [PubMed]
- Tajeddine, N.; Gailly, P. TRPC1 protein channel is major regulator of epidermal growth factor receptor signaling. J. Biol. Chem. 2012, 287, 16146–16157. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Milevskiy, M.J.G.; Kaemmerer, E.; Turner, D.; Yapa, K.T.D.S.; Brown, M.A.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. TRPC1 is a differential regulator of hypoxia-mediated events and Akt signalling in PTEN-deficient breast cancer cells. J. Cell Sci. 2017, 130, 2292–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellini, F.; Sassoli, C.; Nosi, D.; Deledda, C.; Tonelli, P.; Zecchi-Orlandini, S.; Formigli, L.; Giannelli, M. Low pulse energy Nd:YAG laser irradiation exerts a biostimulative effect on different cells of the oral microenvironment: “An in vitro study”. Lasers Surg. Med. 2010, 42, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gu, W.; Zhang, G.; Li, X.; Guo, X. Activation of Notch1 signaling alleviates dysfunction of bone marrow-derived mesenchymal stem cells induced by cigarette smoke extract. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3133–3147. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Kitamura, C.; Fukushima, H.; Nakamichi, I.; Abiko, Y.; Terashita, M.; Jimi, E. Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J. Cell. Biochem. 2010, 111, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Abbasnia, E.; Fathi, M.; Sahraei, H.; Fathi, Y.; Kaka, G. The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts—An in vitro study. Lasers Med. Sci. 2012, 27, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.J.; Song, W.W.; Kim, I.R.; Park, B.S.; Kim, C.H.; Shin, S.H.; Chung, I.K.; Kim, Y.D. Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med. Sci. 2013, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Park, B.S.; Kim, I.R.; Sung, I.Y.; Cho, Y.C.; Kim, J.S.; Kim, Y.D. A novel combination treatment to stimulate bone healing and regeneration under hypoxic conditions: Photobiomodulation and melatonin. Lasers Med. Sci. 2017, 32, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The Effects of Photobiomodulation of 808 nm Diode Laser Therapy at Higher Fluence on the in Vitro Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Bayram, H.; Kenar, H.; Taşar, F.; Hasırcı, V. Effect of low level laser therapy and zoledronate on the viability and ALP activity of Saos-2 cells. Int. J. Oral Maxillofac. Surg. 2013, 42, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.Y.; Wang, Y.M.; Lyu, P.; Hamblin, M.R. Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim. Biophys. Acta 2017, 1861, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Emes, Y.; Akça, K.; Aybar, B.; Yalçın, S.; Çavuşoğlu, Y.; Baysal, U.; Işsever, H.; Atalay, B.; Vural, P.; Ergüven, M.; et al. Low-level laser therapy vs. pulsed electromagnetic field on neonatal rat calvarial osteoblast-like cells. Lasers Med. Sci. 2013, 28, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Pagin, M.T.; de Oliveira, F.A.; Oliveira, R.C.; Sant’Ana, A.C.; de Rezende, M.L.; Greghi, S.L.; Damante, C.A. Laser and light-emitting diode effects on pre-osteoblast growth and differentiation. Lasers Med. Sci. 2014, 29, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bouvet-Gerbettaz, S.; Merigo, E.; Rocca, J.P.; Carle, G.F.; Rochet, N. Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers Surg. Med. 2009, 41, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kharkwal, G.B.; Sajo, M.; Huang, Y.Y.; De Taboada, L.; McCarthy, T.; Hamblin, M.R. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg. Med. 2011, 43, 851–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassoli, C.; Vallone, L.; Tani, A.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: New therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018, 372, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Chellini, F.; Giannelli, M.; Tani, A.; Ballerini, L.; Vallone, L.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Mesenchymal stromal cell and osteoblast responses to oxidized titanium surfaces pre-treated with λ = 808 nm GaAlAs diode laser or chlorhexidine: In vitro study. Lasers Med. Sci. 2017, 32, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Quickenden, T.I.; Daniels, L.L. Attempted biostimulation of division in Saccharomyces cerevisiae using red coherent light. Photochem. Photobiol. 1993, 57, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Lasagni, M.; Bani, D. Thermal effects of λ = 808 nm GaAlAs diode laser irradiation on different titanium surfaces. Lasers Med. Sci. 2015, 30, 2341–2352. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Bani, D.; Tani, A.; Materassi, F.; Chellini, F.; Sassoli, C. Effects of an Erbium:Yttrium-Aluminum-Garnet Laser and Ultrasonic Scaler on Titanium Dioxide-Coated Titanium Surfaces Contaminated with Subgingival Plaque: An In Vitro Study to Assess Post-Treatment Biocompatibility with Osteogenic Cells. J. Periodontol. 2017, 88, 1211–1220. [Google Scholar] [CrossRef] [PubMed]








| Diode Laser/LED Beam Characteristics | |||
|---|---|---|---|
| Diode Laser 635 | GaAlAs Diode Laser 808 | LED 405 | |
| Wavelength (λ) | 635 ± 5 nm | 808 ± 10 nm | 405 ± 5 nm |
| Irradiation mode | continuous wave | continuous wave | continuous wave |
| Handpiece type | Focalized zoom handpiece | Fiber NA = 0.22 | Light pipe glass |
| Applicator Diameter | 0.6 mm | 10 mm | |
| Beam Power | 89 mW | 400 mW | 500 mW |
| Surface treatment data | |||
| Treatment mode | Non-contact | Non-contact | Non-contact |
| Distance from the target | 30 mm | 66 mm | 11 mm |
| Target diameter/surface area | 30 mm/706.9 mm2 18 mm/273 mm2 18 mm/273 mm2 | 30 mm/706.9 mm2 18 mm/273 mm2 | 30 mm/706.9 mm2 18 mm/273 mm2 |
| Exposition time | 30 s | 30 s | 30 s |
| Power density | 12.59 mW/cm2 | 12.59 mW/cm2 | 12.59 mW/cm2 |
| Energy density at target level | 0.378 J/cm2 | 0.378 J/cm2 | 0.378 J/cm2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. https://doi.org/10.3390/ijms19071946
Tani A, Chellini F, Giannelli M, Nosi D, Zecchi-Orlandini S, Sassoli C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. International Journal of Molecular Sciences. 2018; 19(7):1946. https://doi.org/10.3390/ijms19071946
Chicago/Turabian StyleTani, Alessia, Flaminia Chellini, Marco Giannelli, Daniele Nosi, Sandra Zecchi-Orlandini, and Chiara Sassoli. 2018. "Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study" International Journal of Molecular Sciences 19, no. 7: 1946. https://doi.org/10.3390/ijms19071946
APA StyleTani, A., Chellini, F., Giannelli, M., Nosi, D., Zecchi-Orlandini, S., & Sassoli, C. (2018). Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. International Journal of Molecular Sciences, 19(7), 1946. https://doi.org/10.3390/ijms19071946