Early Transcriptomic Response to Phosphate Deprivation in Soybean Leaves as Revealed by RNA-Sequencing
Abstract
:1. Introduction
2. Results
2.1. Transcriptome Profiling by RNA-Seq of Soybean Leaves in Response to Short-Term Pi Deprivation
2.2. Verification of RNA-Seq Data by qRT-PCR
2.3. Enrichment Analysis of Gene Ontology (GO) Functional Annotation and KEGG Pathways of Differentially Expressed Genes (DEGs)
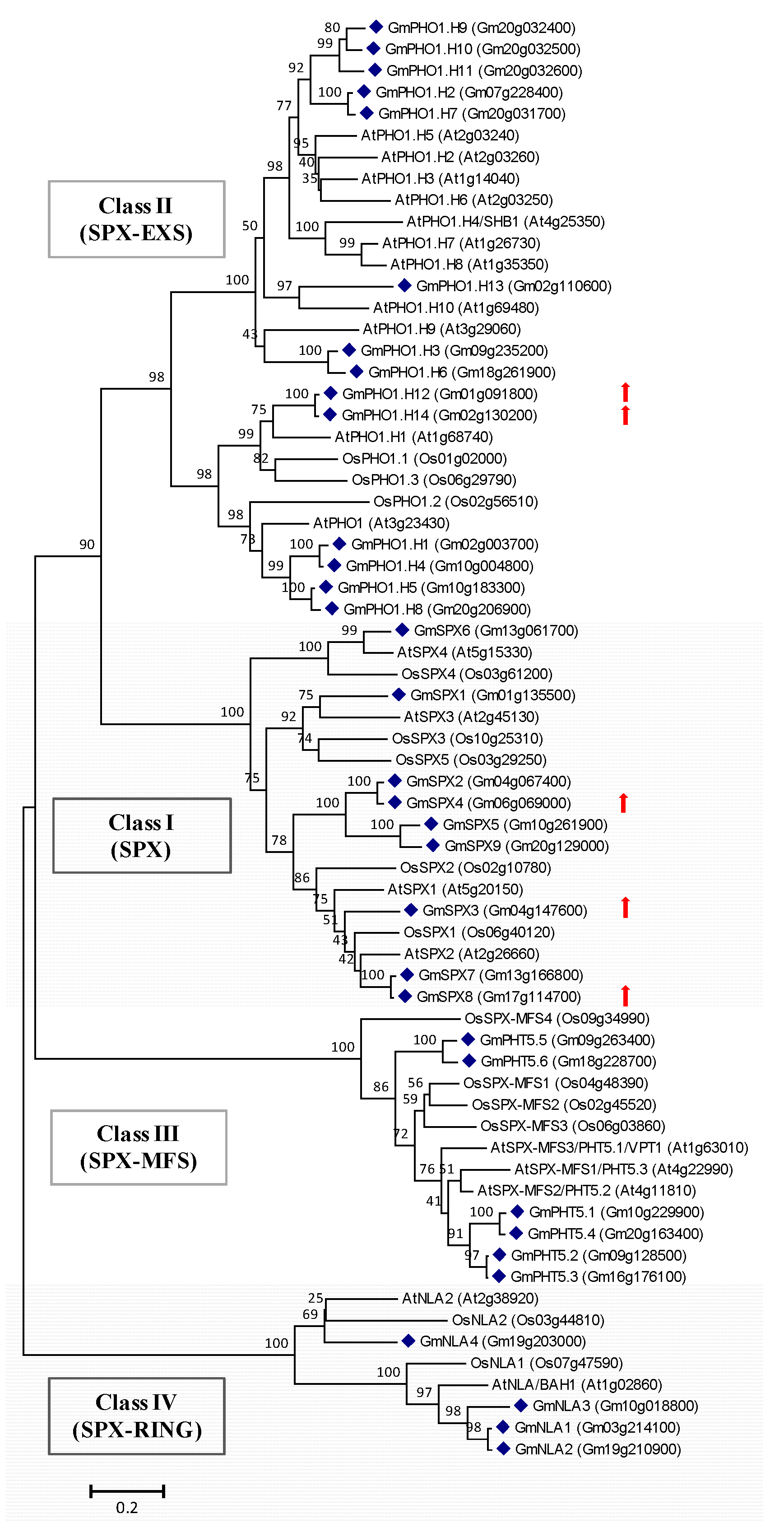
2.4. Genes Potentially Involved in Pi Signaling and Utilization Are Induced by Short-Term Pi Deprivation in Soybean Leaves
2.5. Expression of Genes Potentially Involved in Transportation of Water, Sugars and Mineral Nutrients Is Altered by Short-Term Pi Deprivation
2.6. Genes Linked to Ca2+ and Hormonal Signaling are Regulated by Short-Term Pi Stress
2.7. Diverse Transcription Factor Family Genes Are Responsive to Short-Term Pi Deprivation in Soybean Leaves
2.8. Short-Term Pi Deprivation Modifies the Expression of Genes Encoding Diverse Protein Kinases and Phosphatases
2.9. The Expression of Genes Associated with Metabolism Is Affected by Short-Term Pi Deprivation in Soybean Leaves
2.10. Identification of Pi-Responsive Cis-Regulatory Elements in the Promoters of DEGs
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Pi Concentration Determination
4.3. RNA Isolation, Library Construction and RNA Sequencing
4.4. RNA-Seq Reads Mapping and Differential Counting
4.5. Quantitative RT-PCR Analysis
4.6. Functional Annotation and Gene Ontology (GO) Enrichment
4.7. Promoter Cis-Element Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.M.; Miles, J.W.; Beebe, S.E.; Horst, W.J. Root adaptations to soils with low fertility and aluminium toxicity. Ann. Bot. 2016, 118, 593–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; Lopez-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J.; Lin, S.I. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 2011, 62, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Arredondo, D.L.; Leyva-Gonzalez, M.A.; Gonzalez-Morales, S.I.; Lopez-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shou, H.; Xu, G.; Lian, X. Improvement of phosphorus efficiency in rice on the basis of understanding phosphate signaling and homeostasis. Curr. Opin. Plant Biol. 2013, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, A.; Sun, S.; Xu, G. Complex Regulation of Plant Phosphate Transporters and the Gap between Molecular Mechanisms and Practical Application: What Are Missing? Mol. Plant 2016, 9, 396–416. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Finnegan, P.M.; Jost, R.; Plaxton, W.C.; Shane, M.W.; Stitt, M. Phosphorus nutrition in Proteaceae and beyond. Nat. Plants 2015, 1, 15109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Song, L.; Zhang, Y.; Zheng, Z.; Liu, D. Arabidopsis PHL2 and PHR1 Act Redundantly as the Key Components of the Central Regulatory System Controlling Transcriptional Responses to Phosphate Starvation. Plant Physiol. 2016, 170, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; Linhares, F.; Solano, R.; Martin, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, B.D.; Pant, P.; Erban, A.; Huhman, D.; Kopka, J.; Scheible, W.R. Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant Cell Environ. 2015, 38, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Luan, M.; Wang, Y.; Zhang, C.; Zhang, B.; Shi, J.; Zhao, F.G.; Lan, W.; Luan, S. A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, E6571–E6578. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Huang, T.K.; Yang, S.Y.; Hong, Y.T.; Huang, S.M.; Wang, F.N.; Chiang, S.F.; Tsai, S.Y.; Lu, W.C.; Chiou, T.J. Identification of plant vacuolar transporters mediating phosphate storage. Nat. Commun. 2016, 7, 11095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yue, W.; Ying, Y.; Wang, S.; Secco, D.; Liu, Y.; Whelan, J.; Tyerman, S.D.; Shou, H. Rice SPX-Major Facility Superfamily3, a Vacuolar Phosphate Efflux Transporter, Is Involved in Maintaining Phosphate Homeostasis in Rice. Plant Physiol. 2015, 169, 2822–2831. [Google Scholar] [PubMed]
- Zeng, H.; Wang, G.; Hu, X.; Wang, H.; Du, L.; Zhu, Y. Role of microRNAs in plant responses to nutrient stress. Plant Soil 2014, 374, 1005–1021. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, H.Q.; Song, J.; Feng, S.J.; Yang, Z.M. miRNA778 and SUVH6 are involved in phosphate homeostasis in Arabidopsis. Plant Sci. 2015, 238, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Huang, T.K.; Chiou, T.J. Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 2013, 25, 4061–4074. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; Bennett, M.J.; Bowen, H.C.; Broadley, M.R.; Eastwood, D.C.; May, S.T.; Rahn, C.; Swarup, R.; Woolaway, K.E.; White, P.J. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol. 2003, 132, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Ma, L.; Hou, X.; Wang, M.; Wu, Y.; Liu, F.; Deng, X.W. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 2003, 132, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morcuende, R.; Bari, R.; Gibon, Y.; Zheng, W.; Pant, B.D.; Blasing, O.; Usadel, B.; Czechowski, T.; Udvardi, M.K.; Stitt, M.; et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007, 30, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Jabnoune, M.; Walker, H.; Shou, H.; Wu, P.; Poirier, Y.; Whelan, J. Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. Plant Cell 2013, 25, 4285–4304. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xie, W.; Zhu, T.; Lian, X. Transcriptome response to phosphorus starvation in rice. Acta Physiol. Plant. 2012, 34, 327–341. [Google Scholar] [CrossRef]
- Oono, Y.; Kobayashi, F.; Kawahara, Y.; Yazawa, T.; Handa, H.; Itoh, T.; Matsumoto, T. Characterisation of the wheat (Triticum aestivum L.) transcriptome by de novo assembly for the discovery of phosphate starvation-responsive genes: Gene expression in Pi-stressed wheat. BMC Genom. 2013, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mu, C.; Chen, Y.; Kong, X.; Xu, Y.; Zheng, H.; Zhang, H.; Wang, Q.; Xue, Y.; Li, Z.; et al. Comparative transcript profiling of maize inbreds in response to long-term phosphorus deficiency stress. Plant Physiol. Biochem. 2016, 109, 467–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rourke, J.A.; Yang, S.S.; Miller, S.S.; Bucciarelli, B.; Liu, J.; Rydeen, A.; Bozsoki, Z.; Uhde-Stone, C.; Tu, Z.J.; Allan, D.; et al. An RNA-Seq transcriptome analysis of orthophosphate-deficient white lupin reveals novel insights into phosphorus acclimation in plants. Plant Physiol. 2013, 161, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, M.E.; Plaxton, W.C. Metabolic Adaptations of Plant Respiration to Nutritional Phosphate Deprivation. Plant Physiol. 1993, 101, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, I.M.; Terry, N. Leaf Phosphate Status, Photosynthesis, and Carbon Partitioning in Sugar Beet (IV. Changes with Time Following Increased Supply of Phosphate to Low-Phosphate Plants). Plant Physiol. 1995, 107, 1313–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yan, X.; Liao, H. Genetic improvement for phosphorus efficiency in soybean: A radical approach. Ann. Bot. 2010, 106, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Song, H.; Cheng, H.; Hao, D.; Wang, H.; Kan, G.; Jin, H.; Yu, D. The Acid Phosphatase-Encoding Gene GmACP1 Contributes to Soybean Tolerance to Low-Phosphorus Stress. PLoS Genet. 2014, 10, e1004061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, H.; Chu, S.; Li, H.; Chi, Y.; Triebwasser-Freese, D.; Lv, H.; Yu, D. Integrating QTL mapping and transcriptomics identifies candidate genes underlying QTLs associated with soybean tolerance to low-phosphorus stress. Plant Mol. Biol. 2017, 93, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhao, J.; Tian, J.; Chen, L.; Sun, Z.; Guo, Y.; Lu, X.; Gu, M.; Xu, G.; Liao, H. The high-affinity phosphate transporter GmPT5 regulates phosphate transport to nodules and nodulation in soybean. Plant Physiol. 2012, 159, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Yang, Y.; Du, W.; Zhang, D.; Yu, D.; Cheng, H. A genome-wide expression profile analysis reveals active genes and pathways coping with phosphate starvation in soybean. BMC Genom. 2016, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Q.; Wang, G.P.; Zhang, Y.Q.; Hu, X.Y.; Pi, E.X.; Zhu, Y.Y.; Wang, H.Z.; Du, L.Q. Genome-wide identification of phosphate-deficiency-responsive genes in soybean roots by high-throughput sequencing. Plant Soil 2016, 398, 207–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Ham, B.K.; Chen, J.; Yoshida, A.; Kochian, L.V.; Fei, Z.; Lucas, W.J. Vascular-mediated signalling involved in early phosphate stress response in plants. Nat. Plants 2016, 2, 16033. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-Y.; Ried, M.K.; Hothorn, M.; Poirier, Y. Control of plant phosphate homeostasis by inositol pyrophosphates and the SPX domain. Curr. Opin. Biotechnol. 2018, 49, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Wang, C.; Arpat, B.A.; Wang, Z.; Poirier, Y.; Tyerman, S.D.; Wu, P.; Shou, H.; Whelan, J. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 2012, 193, 842–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Tian, J.; Liao, H. Comparative characterization of GmSPX members reveals that GmSPX3 is involved in phosphate homeostasis in soybean. Ann. Bot. 2014, 114, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Zhao, M.; Wang, Y.; Gai, J.; He, C. Phylogeny, structural evolution and functional diversification of the plant PHOSPHATE1 gene family: A focus on Glycine max. BMC Evol. Biol. 2013, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; Broadley, M.R.; White, P.J. Genetic responses to phosphorus deficiency. Ann. Bot. 2004, 94, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Q.; Zhu, Y.Y.; Huang, S.Q.; Yang, Z.M. Analysis of phosphorus-deficient responsive miRNAs and cis-elements from soybean (Glycine max L.). J. Plant Physiol. 2010, 167, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Mukatira, U.T.; Liu, C.; Varadarajan, D.K.; Raghothama, K.G. Negative regulation of phosphate starvation-induced genes. Plant Physiol. 2001, 127, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gui, S.; Yang, T.; Walk, T.; Wang, X.; Liao, H. Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann. Bot. 2012, 109, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Xu, Y.; Yao, M.; Xie, F.; Gai, J.; Li, Y.; Yang, S. Soybean SPX1 is an important component of the response to phosphate deficiency for phosphorus homeostasis. Plant Sci. 2016, 248, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.X.; Stridh, M.H.; Larsson, K.E.; Liljenberg, C.; Sandelius, A.S. Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 2003, 537, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Cawthray, G.R.; Giavalisco, P.; Kuo, J.; Laliberté, E.; Pearse, S.J.; Scheible, W.-R.; Stitt, M.; Teste, F.; Turner, B.L. Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol. 2012, 196, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramirez, A.; Oropeza-Aburto, A.; Razo-Hernandez, F.; Ramirez-Chavez, E.; Herrera-Estrella, L. Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2006, 103, 6765–6770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essigmann, B.; Guler, S.; Narang, R.A.; Linke, D.; Benning, C. Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1998, 95, 1950–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Xu, C.; Benning, C. Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc. Natl. Acad. Sci. USA 2002, 99, 5732–5737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Welti, R.; Wang, X. Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases D zeta1 and D zeta2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol. 2006, 142, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, M.; Jain, A.; Baldwin, J.C.; Karthikeyan, A.S.; Raghothama, K.G. Characterization of the phosphate starvation-induced glycerol-3-phosphate permease gene family in Arabidopsis. Plant Physiol. 2011, 157, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.I.; Mateos, I.; Charukesi, R.; Wang, Z.; Franco-Zorrilla, J.M.; de Lorenzo, L.; Irigoyen, M.L.; Masiero, S.; Bustos, R.; Rodriguez, J.; et al. SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14947–14952. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhong, Y.; Wang, Y.; Wang, Z.; Zhang, L.; Shi, J.; Wu, Z.; Liu, Y.; Mao, C.; Yi, K.; et al. SPX4 Negatively Regulates Phosphate Signaling and Homeostasis through Its Interaction with PHR2 in Rice. Plant Cell 2014, 26, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Y.; Ruan, W.Y.; Shi, J.; Zhang, L.; Xiang, D.; Yang, C.; Li, C.Y.; Wu, Z.C.; Liu, Y.; Yu, Y.A.; et al. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc. Natl. Acad. Sci. USA 2014, 111, 14953–14958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Wang, Y.; Guo, J.; Zhu, X.; Shi, J.; He, Q.; Liu, Y.; Wu, Y.; Zhang, L.; Lv, Q. Rice SPX6 negatively regulates the phosphate starvation response through suppression of the transcription factor PHR2. New Phytol. 2018, 219, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, A.; Ribot, C.; Rouached, H.; Wang, Y.; Chong, J.; Belbahri, L.; Delessert, S.; Poirier, Y. Members of the PHO1 gene family show limited functional redundancy in phosphate transfer to the shoot, and are regulated by phosphate deficiency via distinct pathways. Plant J. 2007, 50, 982–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radin, J.W. Stomatal responses to water stress and to abscisic acid in phosphorus-deficient cotton plants. Plant Physiol. 1984, 76, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; White, P.J. Sugar signaling in root responses to low phosphorus availability. Plant Physiol. 2011, 156, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, L.; Singh, A.; Wang, H.; Du, L.; Poovaiah, B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 600. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, Y.; Zhang, X.; Pi, E.; Zhu, Y. Analysis of EF-Hand proteins in soybean genome suggests their potential roles in environmental and nutritional stress signaling. Front. Plant Sci. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Poovaiah, B.W.; Du, L.; Wang, H.; Yang, T. Recent advances in calcium/calmodulin-mediated signaling with an emphasis on plant-microbe interactions. Plant Physiol. 2013, 163, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Long, Y.; Schmitz-Thom, I.; Wang, X.-P.; Zhang, C.; Li, H.; Steinhorst, L.; Manishankar, P.; Ren, X.-L.; Offenborn, J.N.; et al. Two spatially and temporally distinct Ca2+ signals convey Arabidopsis thaliana responses to K+ deficiency. New Phytol. 2017, 213, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Riveras, E.; Alvarez, J.M.; Vidal, E.A.; Oses, C.; Vega, A.; Gutierrez, R.A. The Calcium Ion Is a Second Messenger in the Nitrate Signaling Pathway of Arabidopsis. Plant Physiol. 2015, 169, 1397–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Gao, X.; Liao, L.; Harberd, N.P.; Fu, X. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Chiera, J.; Thomas, J.; Rufty, T. Leaf initiation and development in soybean under phosphorus stress. J. Exp. Bot. 2002, 53, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, K.; Wu, Z.; Zhou, J.; Du, L.; Guo, L.; Wu, Y.; Wu, P. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol. 2005, 138, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Li, L.Q.; Xu, Q.; Kong, Y.H.; Wang, H.; Wu, W.H. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 2009, 21, 3554–3566. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, M.; Jain, A.; Raghothama, K.G. Ethylene Response Factor070 regulates root development and phosphate starvation-mediated responses. Plant Physiol. 2014, 164, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Guo, M.; Wu, P.; Yi, K. Phosphate starvation induced OsPHR4 mediates Pi-signaling and homeostasis in rice. Plant Mol. Biol. 2017, 93, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Li, W.; Schmidt, W. Genome-wide co-expression analysis predicts protein kinases as important regulators of phosphate deficiency-induced root hair remodeling in Arabidopsis. BMC Genom. 2013, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Bayle, V.; Arrighi, J.F.; Creff, A.; Nespoulous, C.; Vialaret, J.; Rossignol, M.; Gonzalez, E.; Paz-Ares, J.; Nussaume, L. Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 2011, 23, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.L.; Hurley, B.A.; Tran, H.T.; Valentine, A.J.; She, Y.M.; Knowles, V.L.; Plaxton, W.C. In vivo regulatory phosphorylation of the phosphoenolpyruvate carboxylase AtPPC1 in phosphate-starved Arabidopsis thaliana. Biochem. J. 2009, 420, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wang, Y.; Wang, F.; Yang, J.; Gao, M.; Li, C.; Liu, Y.; Liu, Y.; Yamaji, N.; Ma, J.F.; et al. The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels. Plant Cell 2015, 27, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Gamuyao, R.; Chin, J.H.; Pariasca-Tanaka, J.; Pesaresi, P.; Catausan, S.; Dalid, C.; Slamet-Loedin, I.; Tecson-Mendoza, E.M.; Wissuwa, M.; Heuer, S. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 2012, 488, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, L.; Hong, Y.; Song, F. BOTRYTIS-INDUCED KINASE1, a plasma membrane-localized receptor-like protein kinase, is a negative regulator of phosphate homeostasis in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Li, Y.; Wang, Q.; Xu, J.; Chen, Y.; Yang, H.; Ren, D. Activation of MKK9-MPK3/MPK6 enhances phosphate acquisition in Arabidopsis thaliana. New Phytol. 2014, 203, 1146–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Li, H.-D.; Chen, L.-Q.; Wang, Y.; Liu, L.-L.; He, L.; Wu, W.-H. A Protein Kinase, Interacting with Two Calcineurin B-like Proteins, Regulates K+ Transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zeng, H.; Hu, X.; Zhu, Y.; Chen, Y.; Shen, C.; Wang, H.; Poovaiah, B.W.; Du, L. Identification and expression analyses of calmodulin-binding transcription activator genes in soybean. Plant Soil 2015, 386, 205–221. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Poree, F.; Nagel, A.; Lohse, M.; Czedik-Eysenberg, A.; Stitt, M. A guide to using MapMan to visualize and compare Omics data in plants: A case study in the crop species, Maize. Plant Cell Environ. 2009, 32, 1211–1229. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rivera, A.; Defrance, M.; Sand, O.; Herrmann, C.; Castro-Mondragon, J.A.; Delerce, J.; Jaeger, S.; Blanchet, C.; Vincens, P.; Caron, C.; et al. RSAT 2015: Regulatory Sequence Analysis Tools. Nucleic Acids Res. 2015, 43, W50–W56. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Accession No. | −P (FPKM) | +P (FPKM) | p Value | Description |
|---|---|---|---|---|
| Gm04g223400 | 1260.24 | 0.00 | 1.98 × 10−2 | NA |
| Gm17g100000 | 21.71 | 0.00 | 1.65 × 10−3 | mini zinc finger |
| Gm17g090400 | 3.82 | 0.00 | 3.95× 10−3 | calcium-dependent protein kinase 6 |
| Gm16g028300 | 2.72 | 0.00 | 4.10 × 10−2 | NA |
| Gm15g099200 | 2.27 | 0.00 | 2.00 × 10−4 | NA |
| Gm06g240900 | 1.25 | 0.00 | 1.50 × 10−2 | PLATZ transcription factor family protein |
| Gm09g023000 | 1.11 | 0.00 | 5.00 × 10−5 | peroxidase superfamily protein |
| Gm06g177200 | 0.77 | 0.00 | 2.40 × 10−3 | NA |
| Gm19g246600 | 0.75 | 0.00 | 1.00 × 10−4 | SSXT family protein |
| Gm13g068300 | 0.00 | 47.11 | 9.65 × 10−3 | NA |
| Gm05g099700 | 0.00 | 10.71 | 3.85 × 10−3 | NA |
| Gm04g128500 | 0.00 | 5.87 | 7.50 × 10−4 | late embryogenesis abundant protein, group 1 protein |
| Gm13g236300 | 0.00 | 5.77 | 9.00 × 10−4 | NA |
| Gm04g014000 | 0.00 | 3.30 | 5.00 × 10−5 | polyol/monosaccharide transporter 5 |
| Gm13g178000 | 0.00 | 2.18 | 4.19 × 10−2 | NA |
| Gm07g022900 | 0.00 | 2.11 | 8.65 × 10−3 | phospholipid N-methyltransferase |
| Gm04g094800 | 0.00 | 1.21 | 5.00 × 10−5 | plant-specific transcription factor YABBY family protein |
| Gm11g179100 | 0.00 | 1.02 | 5.00 × 10−5 | aluminum activated malate transporter family protein |
| Gm03g113600 | 0.00 | 0.82 | 5.00 × 10−5 | auxin efflux carrier family protein |
| Gm04g044300 | 0.00 | 0.80 | 1.61 × 10−2 | cytochrome B5 isoform D |
| Gm15g197300 | 0.00 | 0.73 | 5.00 × 10−5 | CBL-interacting protein kinase 23 |
| Accession No. | −P (FPKM) | +P (FPKM) | Log2(−P/+P) | p Value | Description |
|---|---|---|---|---|---|
| Gm03g078300 | 1.59 | 0.09 | 4.15 | 4.42 × 10−2 | Sulfoquinovosyldiacylglycerol 2 (GmSQD2) |
| Gm04g147600 | 2.80 | 0.41 | 2.76 | 1.03 × 10−2 | SPX domain-containing protein 1-related (GmSPX3) |
| Gm08g056400 | 9.38 | 1.43 | 2.71 | 5.00 × 10−5 | Purple acid phosphatase (GmPAP13) |
| Gm01g091800 | 0.90 | 0.22 | 2.02 | 4.90 × 10−3 | Phosphate transporter PHO1 homolog 1 (GmPHO1;H12) |
| Gm02g130200 | 1.28 | 0.32 | 2.02 | 6.50 × 10−4 | Phosphate transporter PHO1 homolog 1 (GmPHO1;H14) |
| Gm20g238000 | 1.42 | 0.38 | 1.89 | 1.00 × 10−4 | Phospholipase D P1 (GmPLDZ2) |
| Gm06g069000 | 2.68 | 0.91 | 1.56 | 2.52 × 10−2 | SPX domain-containing protein 1-related (GmSPX4) |
| Gm17g114700 | 19.96 | 7.53 | 1.41 | 5.00 × 10−5 | SPX domain-containing protein 1-related (GmSPX8) |
| Gm08g194100 | 0.71 | 0.29 | 1.29 | 2.20 × 10−2 | Phospholipase D α 4 |
| Gm01g002400 | 13.80 | 6.68 | 1.05 | 1.31 × 10−2 | Phospholipase A2 family protein |
| Gm09g223700 | 34.43 | 16.85 | 1.03 | 5.00 × 10−5 | Glycerol-3-phosphate permease gene family |
| Gm19g026600 | 2.78 | 1.16 | 1.27 | 1.40 × 10−3 | Purple acid phosphatase (GmPAP31) |
| Gm16g220900 | 2.14 | 4.75 | −1.15 | 1.64 × 10−2 | HAD superfamily, subfamily IIIB acid phosphatase |
| Substrate | Accession No. | −P (FPKM) | +P (FPKM) | Log2(−P/+P) | p Value | Description |
|---|---|---|---|---|---|---|
| Sugar | Gm15g105900 | 1.48 | 0.47 | 1.64 | 2.78 × 10−2 | Glucose-6-phosphate/phosphate translocator 2 |
| Gm13g206700 | 6.26 | 2.77 | 1.18 | 9.00 × 10−4 | Glucose-6-phosphate/phosphate translocator 2 | |
| Gm11g226200 | 131.29 | 63.50 | 1.05 | 5.00 × 10−5 | Nucleotide-sugar transporter family protein | |
| Gm04g014000 | 0.00 | 3.30 | +P only | 5.00 × 10−5 | Polyol/monosaccharide transporter 5 | |
| Gm08g009900 | 2.80 | 19.84 | −2.83 | 5.00 × 10−5 | Bidirectional sugar transporter SWEET10 | |
| Zinc/iron | Gm16g168200 | 7.32 | 2.14 | 1.78 | 1.40 × 10−3 | Vacuolar iron transporter (VIT) family protein |
| Gm13g004400 | 1.33 | 7.82 | −2.55 | 5.00 × 10−5 | Zinc transporter 3-related protein | |
| Gm20g063100 | 1.80 | 9.06 | −2.33 | 5.00 × 10−5 | Zinc transporter 3-related protein | |
| Gm06g052000 | 24.56 | 58.72 | −1.26 | 5.00 × 10−5 | Zinc/iron transporter | |
| Sulfate | Gm01g175200 | 2.16 | 0.83 | 1.37 | 3.55 × 10−3 | Sulfite exporter TauE/SafE family protein |
| Gm15g014000 | 1.78 | 0.84 | 1.08 | 1.60 × 10−2 | Sulfate transporter 3;4 | |
| Copper | Gm08g180500 | 4.15 | 0.81 | 2.36 | 4.03 × 10−2 | Copper transport protein ATOX1-related |
| Gm14g072100 | 6.81 | 2.58 | 1.40 | 5.00 × 10−5 | Copper transport protein ATOX1-related | |
| Gm15g051900 | 28.02 | 13.36 | 1.07 | 1.85 × 10−3 | Copper transport protein ATOX1-related | |
| Nitrate | Gm18g127200 | 1.07 | 3.08 | −1.53 | 4.50 × 10−4 | Nitrate transporter 1.7 |
| Malate | Gm11g179100 | 0.00 | 1.02 | +P only | 5.00 × 10−5 | Aluminum activated malate transporter |
| Water | Gm08g015300 | 8.72 | 3.58 | 1.29 | 3.50 × 10−4 | Aquaporin PIP1;4-related |
| Gm05g208700 | 15.22 | 6.50 | 1.23 | 1.50 × 10−4 | Aquaporin PIP1;4-related | |
| Gm15g018100 | 69.65 | 33.30 | 1.06 | 5.00 × 10−5 | Aquaporin TIP2;1 | |
| Unknown | Gm07g081000 | 18.37 | 4.44 | 2.05 | 5.00 × 10−5 | ABC transporter family protein |
| Gm11g106300 | 54.15 | 17.68 | 1.61 | 5.00 × 10−5 | Major facilitator superfamily protein | |
| Gm04g027800 | 1.28 | 0.35 | 1.86 | 2.71 × 10−2 | Protein Walls Are Thin 1 | |
| Gm07g094000 | 0.43 | 2.97 | −2.77 | 5.30 × 10−3 | ATP-binding cassette A2 | |
| Gm11g064100 | 0.87 | 2.05 | −1.23 | 2.15 × 10−3 | Major facilitator superfamily protein | |
| Gm08g101500 | 1.01 | 2.36 | −1.22 | 5.00 × 10−5 | Multidrug resistance-associated protein 3 | |
| Gm17g165200 | 5.68 | 11.51 | −1.02 | 5.00 × 10−5 | Metal transporter NRAMP2-related | |
| Gm10g276700 | 2.39 | 4.79 | −1.00 | 6.00 × 10−4 | Major facilitator superfamily protein |
| Ca2+/Hormone | Accession No. | −P (FPKM) | +P (FPKM) | Log2(−P/+P) | p Value | Description |
|---|---|---|---|---|---|---|
| Calcium | Gm02g226500 | 1.10 | 0.24 | 2.19 | 2.14 × 10−2 | Ca2+/H+ antiporter VCX1 and related protein |
| Gm14g000100 | 1.59 | 6.01 | −1.92 | 5.00 × 10−5 | Glutamate receptor 3.1-related protein | |
| Gm16g061800 | 0.37 | 0.83 | −1.15 | 1.52 × 10−2 | Glutamate receptor 2.5 | |
| Gm07g203700 | 1.58 | 3.28 | −1.06 | 2.50 × 10−4 | Glutamate receptor 2.7 | |
| Gm17g090400 | 3.82 | 0.00 | −P only | 3.95 × 10−3 | Calcium-dependent protein kinase 6 | |
| Gm17g130900 | 4.20 | 1.38 | 1.60 | 5.00 × 10−4 | Calmodulin-binding protein IQ-domain 22 | |
| Gm09g225300 | 1.10 | 0.37 | 1.57 | 1.30 × 10−2 | Protein IQ-domain 11 | |
| Gm01g002400 | 13.80 | 6.68 | 1.05 | 1.31 × 10−2 | Phospholipase A2 family protein | |
| Gm18g230800 | 1.07 | 2.74 | −1.36 | 5.00 × 10−5 | Ca2+/lipid-binding phosphoribosyltransferase | |
| Gm09g236800 | 8.07 | 16.28 | −1.01 | 1.70 × 10−3 | Ca2+-binding EF-hand family protein (GmCML116) | |
| Auxin | Gm16g115500 | 2.69 | 1.15 | 1.23 | 1.65 × 10−2 | Auxin efflux carrier family protein |
| Gm03g113600 | 0.00 | 0.82 | +P only | 5.00 × 10−5 | Auxin efflux carrier family protein | |
| Gm19g168500 | 0.50 | 1.59 | −1.66 | 2.75 × 10−2 | Auxin-responsive protein IAA10-related | |
| Gm19g161100 | 9.82 | 25.41 | −1.37 | 5.00 × 10−5 | AUX/IAA family | |
| Cytokinin | Gm04g247800 | 3.32 | 1.03 | 1.70 | 1.80 × 10−2 | Response regulator of two-component system |
| Gm05g144500 | 0.46 | 1.09 | −1.22 | 1.58 × 10−2 | Response regulator of two-component system | |
| Gibberellin | Gm06g044400 | 439.61 | 89.83 | 2.29 | 5.00 × 10−5 | Gibberellin-regulated family protein |
| Gm06g185300 | 71.42 | 15.60 | 2.19 | 1.00 × 10−4 | Gibberellin-regulated family protein | |
| Gm05g034500 | 19.80 | 6.63 | 1.58 | 1.89 × 10−2 | Gibberellin-regulated family protein | |
| Gm06g193800 | 174.53 | 71.57 | 1.29 | 2.00 × 10−4 | Gibberellin-regulated family protein | |
| Gm17g092800 | 17.65 | 7.92 | 1.16 | 4.94 × 10−2 | Gibberellin-regulated family protein | |
| Gm15g002200 | 5.27 | 2.62 | 1.01 | 1.35 × 10−3 | GA requiring 3 | |
| Brassinosteroid | Gm14g127400 | 1.36 | 0.24 | 2.52 | 1.28 × 10−2 | BES1/BZR1 homolog protein 1 |
| Jasmonate | Gm01g204400 | 4.22 | 1.71 | 1.30 | 4.99 × 10−2 | JASMONATE-ZIM-domain protein 2-related |
| Ethylene | Gm10g008500 | 2.20 | 4.84 | −1.14 | 2.61 × 10−2 | Ethylene response sensor 2-related |
| Accession No. | −P (FPKM) | +P (FPKM) | Log2(−P/+P) | p Value | Description |
|---|---|---|---|---|---|
| Gm14g127400 | 1.36 | 0.24 | 2.52 | 1.28 × 10−2 | BES1/BZR1 homolog protein 1-related |
| Gm17g257700 | 1.25 | 0.31 | 2.00 | 4.86 × 10−2 | C2H2-like zinc finger protein |
| Gm08g215400 | 1.86 | 0.47 | 1.99 | 3.36 × 10−2 | Basic helix-loop-helix (bHLH) DNA-binding superfamily protein |
| Gm06g284900 | 1.93 | 0.54 | 1.82 | 3.15 × 10−3 | Basic-leucine zipper (bZIP) transcription factor family protein |
| Gm16g017700 | 4.93 | 1.56 | 1.66 | 2.50 × 10−4 | Basic helix-loop-helix (bHLH) DNA-binding superfamily protein |
| Gm20g186500 | 12.12 | 4.34 | 1.48 | 5.00 × 10−5 | MYB-like transcription factor family protein |
| Gm12g037200 | 7.12 | 2.78 | 1.36 | 1.59 × 10−2 | B-box type zinc finger family protein |
| Gm07g012100 | 8.87 | 3.59 | 1.30 | 2.10 × 10−3 | DOF zinc finger protein 1 |
| Gm18g042300 | 2.28 | 0.94 | 1.29 | 5.35 × 10−3 | Zinc finger protein 4 |
| Gm13g061900 | 1.19 | 0.51 | 1.22 | 4.94 × 10−2 | MYB domain protein 16 |
| Gm05g056000 | 5.89 | 2.62 | 1.17 | 1.16 × 10−2 | Plant-specific transcription factor YABBY family protein |
| Gm01g133500 | 7.16 | 3.43 | 1.06 | 4.33 × 10−2 | Basic helix-loop-helix (bHLH) DNA-binding superfamily protein |
| Gm11g117100 | 6.84 | 3.35 | 1.03 | 1.05 × 10−3 | Basic helix-loop-helix (bHLH) DNA-binding superfamily protein |
| Gm04g094800 | 0.00 | 1.21 | +P only | 5.00 × 10−5 | Plant-specific transcription factor YABBY family protein |
| Gm13g203700 | 0.13 | 1.03 | −3.02 | 4.61 × 10−2 | C2H2-type zinc finger family protein |
| Gm01g029300 | 1.37 | 6.15 | −2.16 | 3.77 × 10−2 | Plant-specific transcription factor YABBY family protein |
| Gm10g021400 | 0.35 | 1.44 | −2.06 | 2.51 × 10−2 | B-box type zinc finger protein with CCT domain |
| Gm11g182000 | 0.92 | 3.34 | −1.87 | 1.33 × 10−2 | NAC domain containing protein 90 |
| Gm13g047400 | 0.31 | 1.10 | −1.85 | 1.38 × 10−2 | TCP family transcription factor |
| Gm12g162700 | 0.62 | 2.11 | −1.76 | 2.43 × 10−2 | ERF domain protein 9 |
| Gm02g177800 | 0.85 | 2.71 | −1.67 | 3.78 × 10−2 | MYB-like HTH transcriptional regulator family protein |
| Gm05g002700 | 0.29 | 0.89 | −1.62 | 4.98 × 10−2 | NAC (No Apical Meristem) domain transcriptional regulator |
| Gm01g041700 | 1.09 | 2.98 | −1.46 | 7.15 × 10−3 | Homeobox 1 |
| Gm02g217800 | 4.27 | 11.31 | −1.40 | 1.00 × 10−4 | Transcription factor bHLH61-related |
| Gm03g221700 | 1.40 | 3.66 | −1.39 | 2.28 × 10−2 | MYB domain protein 2 |
| Gm08g256400 | 1.43 | 3.54 | −1.31 | 7.20 × 10−3 | TCP family transcription factor |
| Gm02g152900 | 0.94 | 2.25 | −1.25 | 2.90 × 10−2 | B-box type zinc finger protein with CCT domain |
| Gm05g144500 | 0.46 | 1.09 | −1.22 | 1.58 × 10−2 | Response regulator 11 |
| Gm07g227200 | 6.34 | 12.74 | −1.01 | 1.50 × 10−4 | WRKY DNA-binding protein 3 |
| Gm08g286000 | 1.41 | 2.83 | −1.00 | 1.25 × 10−2 | Zinc finger (CCCH-type/C3HC4-type RING finger) family protein |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Zhang, X.; Zhang, X.; Pi, E.; Xiao, L.; Zhu, Y. Early Transcriptomic Response to Phosphate Deprivation in Soybean Leaves as Revealed by RNA-Sequencing. Int. J. Mol. Sci. 2018, 19, 2145. https://doi.org/10.3390/ijms19072145
Zeng H, Zhang X, Zhang X, Pi E, Xiao L, Zhu Y. Early Transcriptomic Response to Phosphate Deprivation in Soybean Leaves as Revealed by RNA-Sequencing. International Journal of Molecular Sciences. 2018; 19(7):2145. https://doi.org/10.3390/ijms19072145
Chicago/Turabian StyleZeng, Houqing, Xiajun Zhang, Xin Zhang, Erxu Pi, Liang Xiao, and Yiyong Zhu. 2018. "Early Transcriptomic Response to Phosphate Deprivation in Soybean Leaves as Revealed by RNA-Sequencing" International Journal of Molecular Sciences 19, no. 7: 2145. https://doi.org/10.3390/ijms19072145





