Genome-Wide Characterization and Analysis of Metallothionein Family Genes That Function in Metal Stress Tolerance in Brassica napus L.
Abstract
:1. Introduction
2. Results
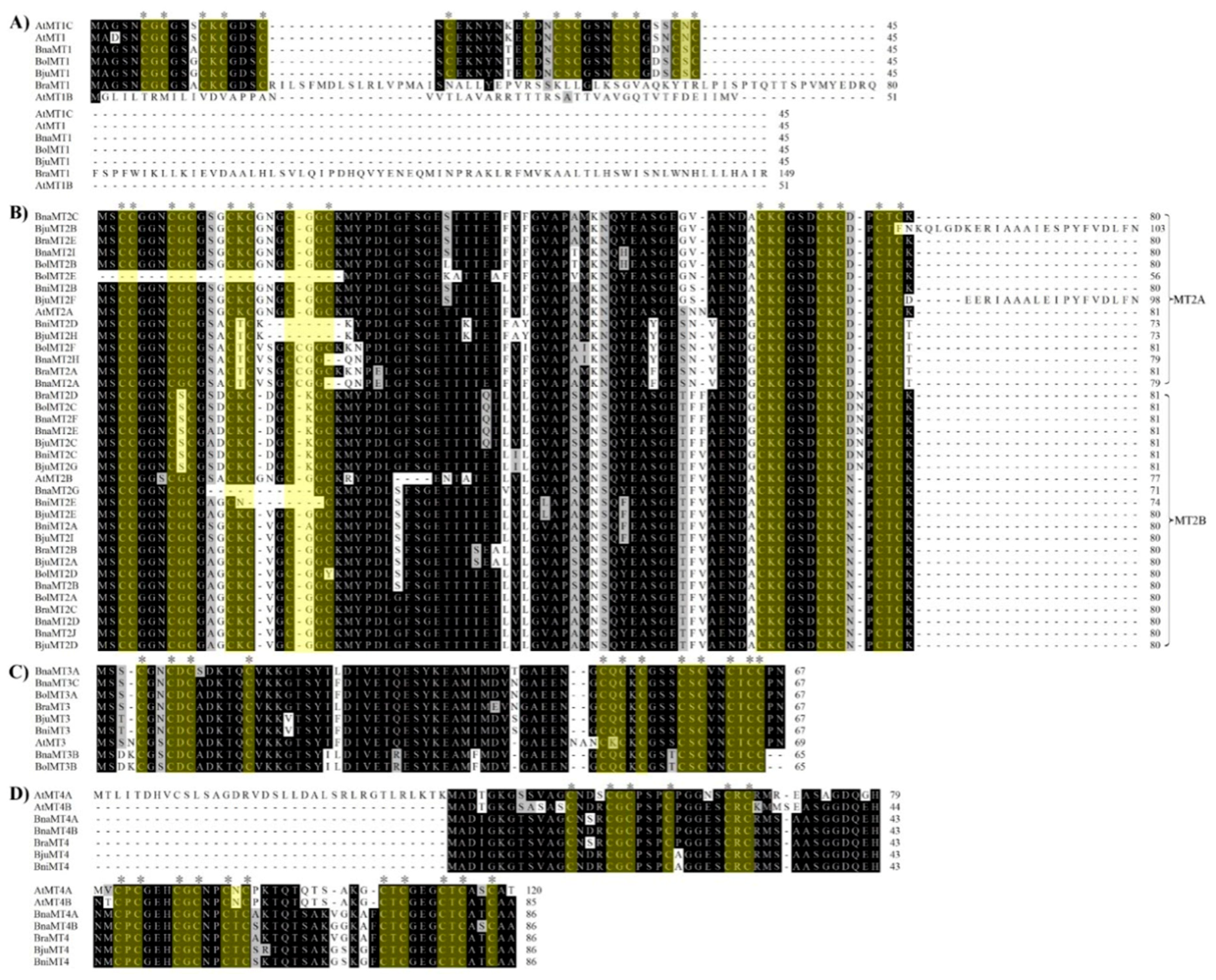
2.1. Identification and Multiple Sequence Alignment of MT Family Genes
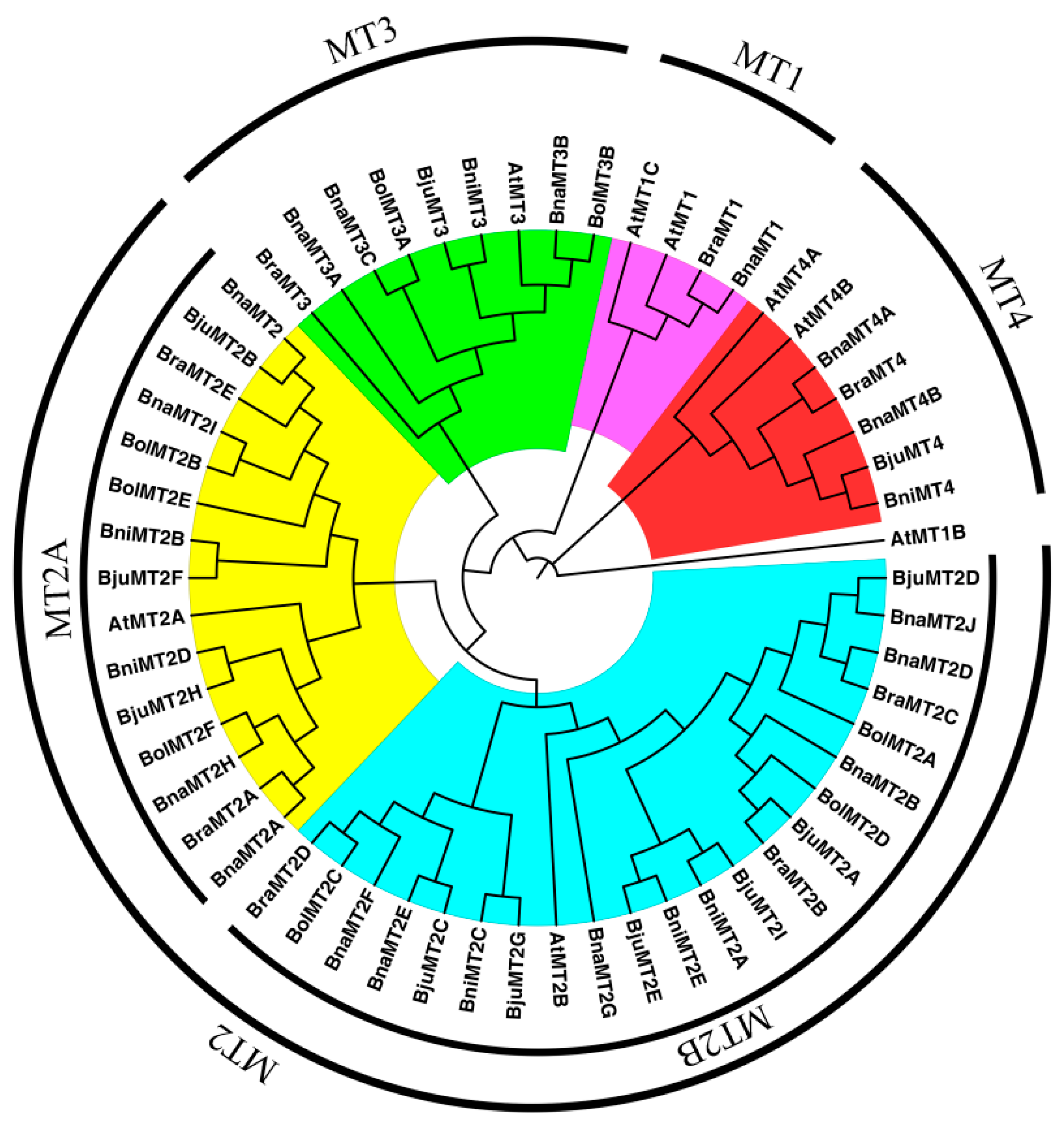
2.2. Phylogenetic Analysis of MT Family Genes
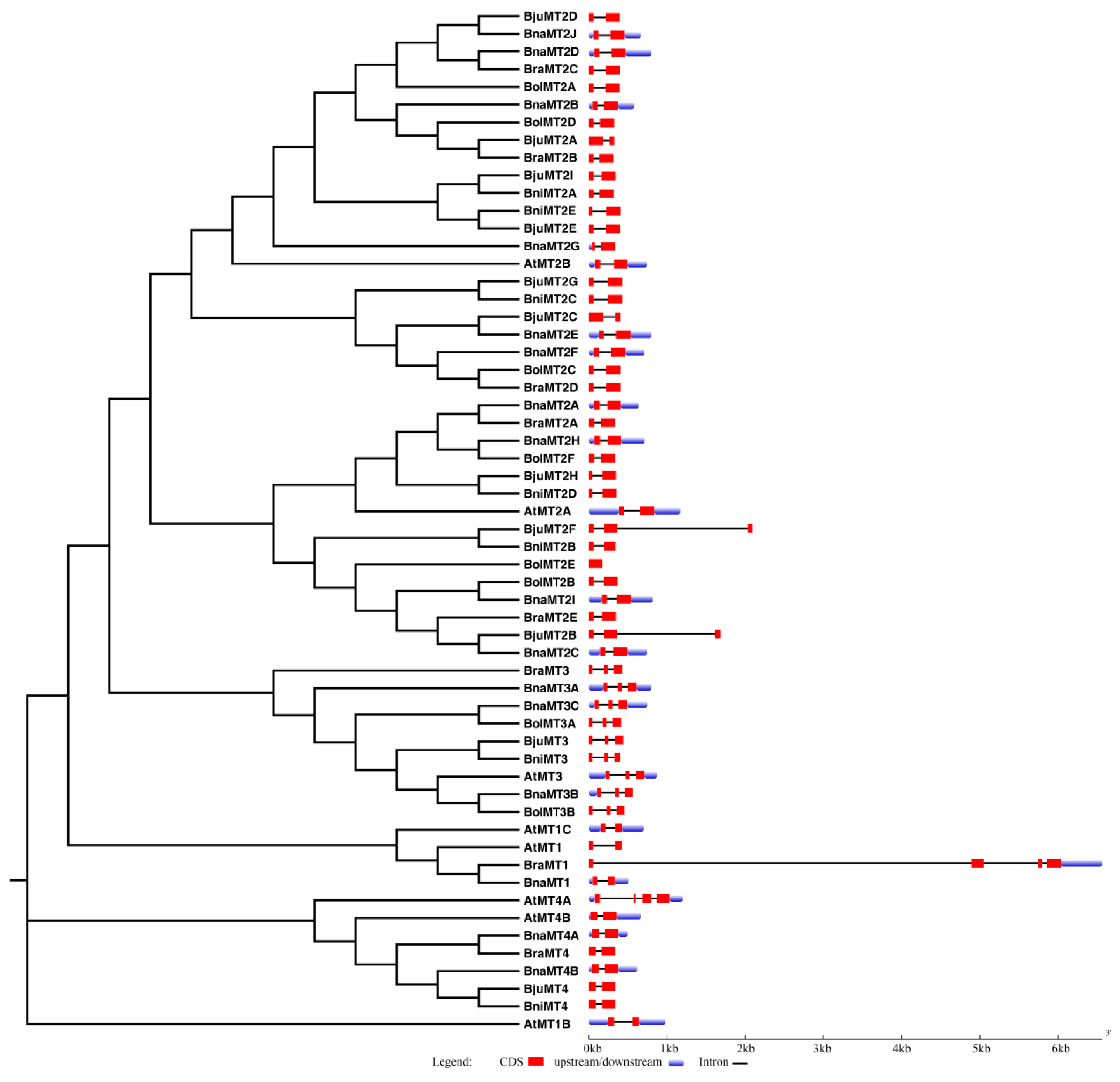
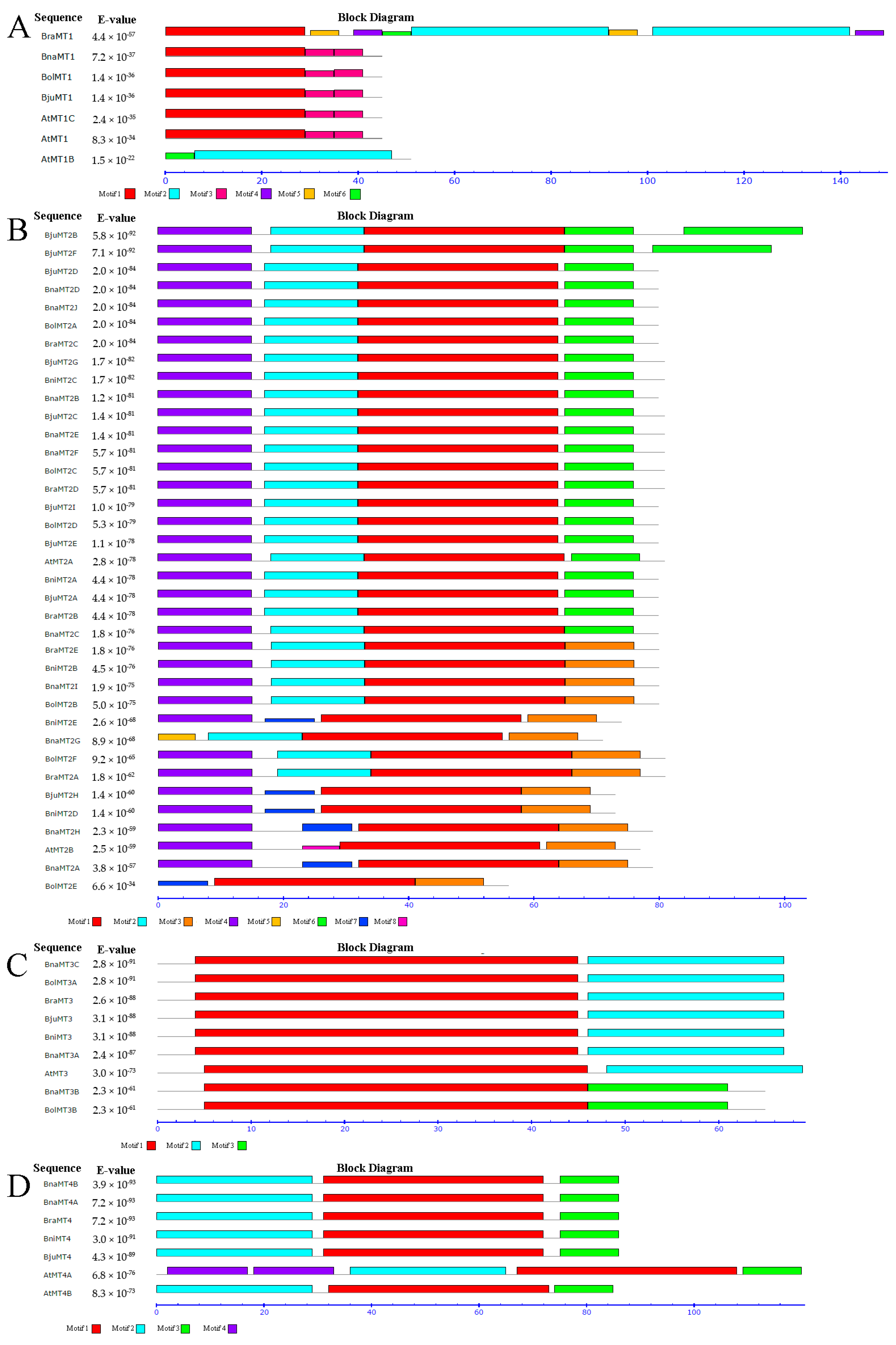
2.3. Genomic Structure and Conserved Motif Analysis of the MT Gene Family
2.4. Chromosome Locations and Duplication of MT Genes in Brassica
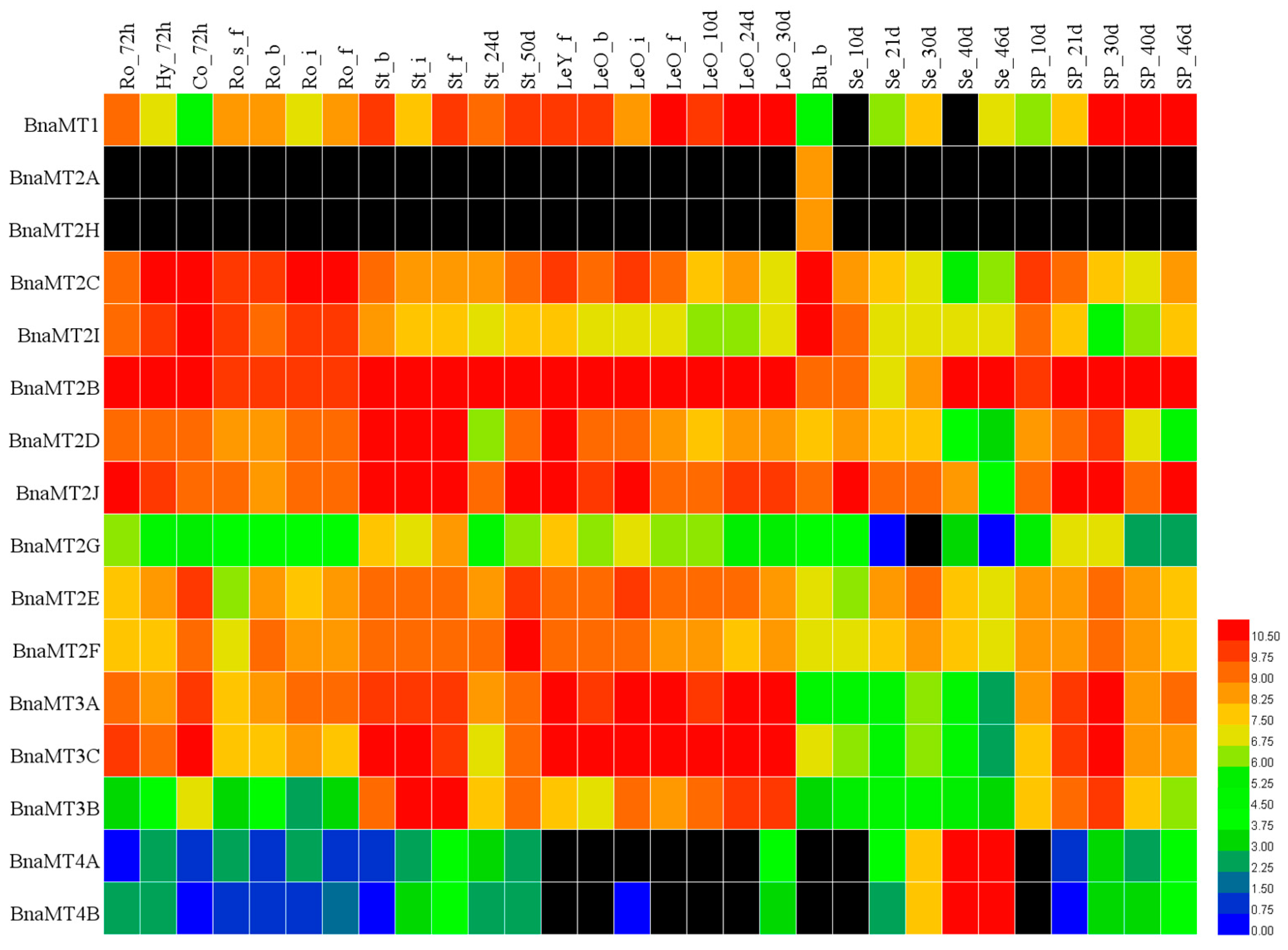
2.5. Expression Profiles of BnaMT Family Genes in B. napus
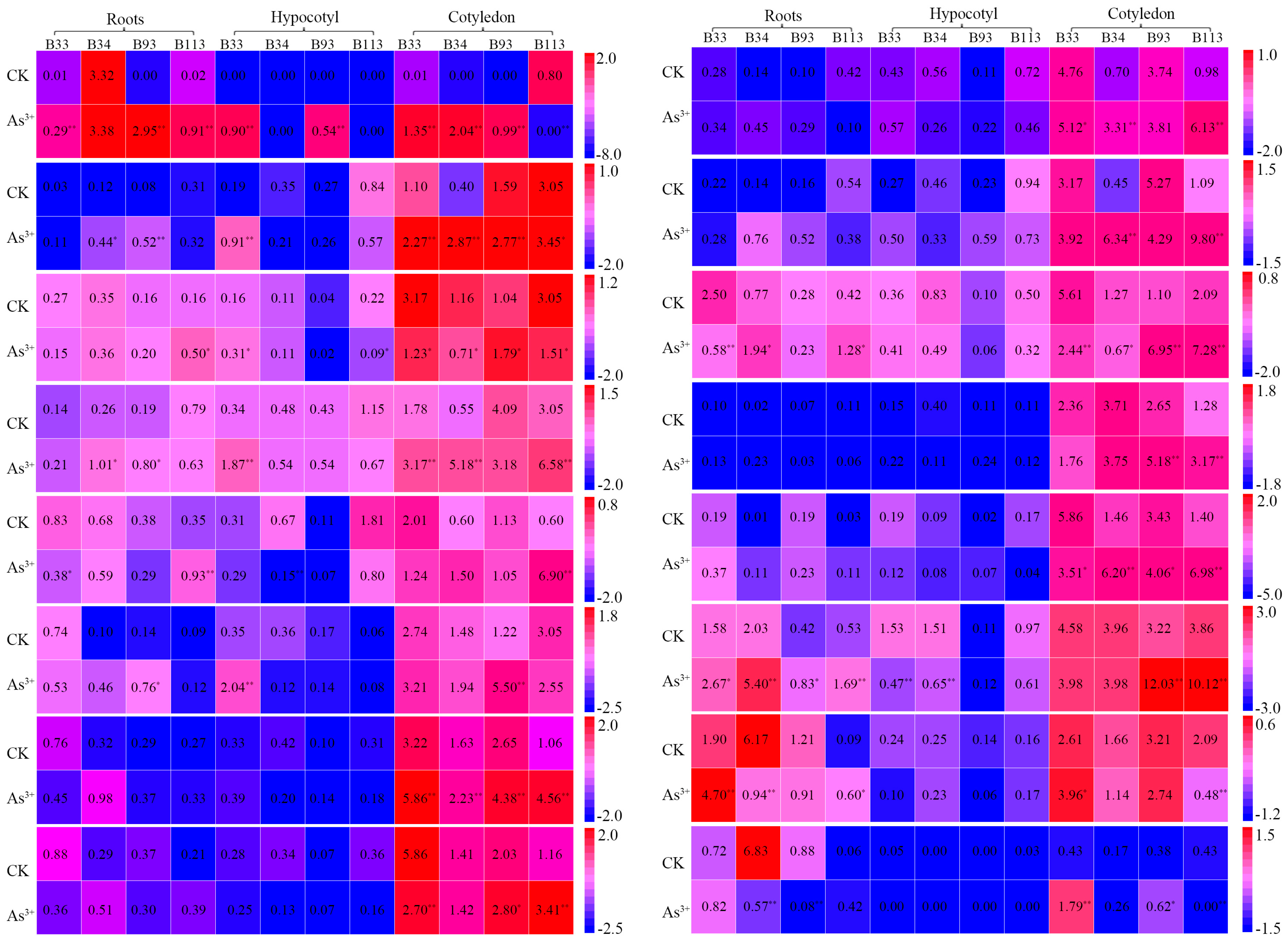
2.6. Expression Analysis of BnaMT Genes in Response to Metal Treatment
3. Discussion
4. Materials and Methods
4.1. Identification of MT Family Genes in Brassica
4.2. Multiple Sequence Alignment and Phylogenetic Analysis of MTs in Brassica
4.3. Conserved Motif Recognition and Gene Structure Analysis
4.4. Chromosomal Locations of MT Family Genes in B. napus
4.5. Plant Materials and Metal Stress Treatments
4.6. Total RNA Extraction and RT-qPCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ebbs, S.; Uchil, S. Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) czern] involves preferential loss of chlorophyll b. Photosynthetica 2008, 46, 49–55. [Google Scholar] [CrossRef]
- Lingua, G.; Franchin, C.; Todeschini, V.; Castiglione, S.; Biondi, S.; Burlando, B.; Parravicini, V.; Torrigiani, P.; Berta, G. Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ. Pollut. 2008, 153, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Deng, X.; Quan, L.; Xia, Y.; Shen, Z. Metallothioneins BcMT1 and BcMT2 from Brassica campestris enhance tolerance to cadmium and copper and decrease production of reactive oxygen species in Arabidopsis thaliana. Plant Soil 2013, 367, 507–519. [Google Scholar] [CrossRef]
- Huang, G.Y.; Wang, Y.S. Expression and characterization analysis of type 2 metallothionein from grey mangrove species (Avicennia marina) in response to metal stress. Aquat. Toxicol. 2010, 99, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Mir, G.; Domenech, J.; Huguet, G.; Guo, W.J.; Goldsbrough, P.; Atrian, S.; Molinas, M. A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. J. Exp. Bot. 2004, 55, 2483–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.S.; Liu, L.Q.; Deng, Y.M.; Zhu, X.D.; Huang, S.Z.; Lu, X.Q. The heterologous expression of the Iris lactea var. chinensis type 2 metallothionein IlMT2b gene enhances copper tolerance in Arabidopsis thaliana. Bull. Environ. Contam. Toxicol. 2015, 94, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hassinen, V.H.; Tuomainen, M.; Peraniemi, S.; Schat, H.; Karenlampi, S.O.; Tervahauta, A.I. Metallothioneins 2 and 3 contribute to the metal-adapted phenotype but are not directly linked to Zn accumulation in the metal hyperaccumulator, Thlaspi caerulescens. J. Exp. Bot. 2009, 60, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Bourdineaud, J.P.; Baudrimont, M.; Gonzalez, P.; Moreau, J.L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.J.; Tommey, A.M.; Kuske, C.; Jackson, P.J. Plant metallothioneins. Biochem. J. 1993, 295, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leszczyszyn, O.I.; Imam, H.T.; Blindauer, C.A. Diversity and distribution of plant metallothioneins: A review of structure, properties and functions. Metallomics 2013, 5, 1146–1169. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.J.; Bundithya, W.; Goldsbrough, P.B. Characterization of the Arabidopsis metallothionein gene family: Tissue-specific expression and induction during senescence and in response to copper. New Phytol. 2003, 159, 369–381. [Google Scholar] [CrossRef]
- Benatti, M.R.; Yookongkaew, N.; Meetam, M.; Guo, W.J.; Punyasuk, N.; Abuqamar, S.; Goldsbrough, P. Metallothionein deficiency impacts copper accumulation and redistribution in leaves and seeds of Arabidopsis. New Phytol. 2014, 202, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.J.; Meetam, M.; Goldsbrough, P.B. Examining the specific contributions of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol. 2008, 146, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shim, D.; Song, W.Y.; Hwang, I.; Lee, Y. Arabidopsis metallothioneins 2a and 3 enhance resistance to cadmium when expressed in Vicia faba guard cells. Plant Mol. Biol. 2004, 54, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, Y.; Chen, H.; Li, G.; Zhang, X.; Zhao, J. Type 4 metallothionein genes are involved in regulating Zn ion accumulation in late embryo and in controlling early seedling growth in Arabidopsis. Plant Cell Environ. 2012, 35, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Roosens, N.; Leplae, R.; Bernard, C.; Verbruggen, N. Variations in plant metallothioneins: The heavy metal hyperaccumulator Thlaspi caerulescens as a study case. Planta 2005, 222, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; Bowen, H.C.; White, P.J.; Mills, V.; Pyke, K.A.; Baker, A.J.; Whiting, S.N.; May, S.T.; Broadley, M.R. A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol. 2006, 170, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, D.; Ren, Y.; Zhang, X.; Zhao, J. Characteristic and Expression Analysis of a Metallothionein Gene, OsMT2b, Down-Regulated by Cytokinin Suggests Functions in Root Development and Seed Embryo Germination of Rice. Plant Physiol. 2008, 146, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, P.; Chen, H.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.; Jiang, L.; Wu, K.; Huang, S. Overexpression of Nelumbo nucifera metallothioneins 2a and 3 enhances seed germination vigor in Arabidopsis. Planta 2012, 235, 523–537. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Li, C.; Zu, Y.; Du, Y.; Andreas, W.; Gromes, R.; Rausch, T. Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. J. Exp. Bot. 2006, 57, 3575–3582. [Google Scholar]
- Kumar, P.B.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 1995, 13, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, P.; Gusiatin, Z.M.; Cretescu, I. Phytoextraction of Cd and Zn as single or mixed pollutants from soil by rape (Brassica napus). Environ. Sci. Pollut. Res. 2016, 23, 10693–10701. [Google Scholar] [CrossRef] [PubMed]
- Gasic, K.; Korban, S.S. Expression of Arabidopsis phytochelatin synthase in Indian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta 2007, 225, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, B.B.M.; Diehl, S.V.; Han, F.X.; Monts, D.L.; Su, Y. Anatomical changes due to uptake and accumulation of Zn and Cd in Indian mustard (Brassica juncea). Environ. Exp. Bot. 2005, 54, 131–141. [Google Scholar] [CrossRef]
- Ishikawa, S.; Noriharu, A.; Murakami, M.; Wagatsuma, T. Is Brassica juncea a suitable plant for phytoremediation of cadmium in soils with moderately low cadmium contamination?—Possibility of using other plant species for Cd-phytoextraction. Soil Sci. Plant Nutr. 2006, 52, 32–42. [Google Scholar] [CrossRef]
- Marchiol, L.; Assolari, S.; Sacco, P.; Zerbi, G. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ. Pollut. 2004, 132, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Solhi, M.; Shareatmadari, H.; Hajabbasi, M.A. Lead and Zinc Extraction Potential of Two Common Crop Plants, Helianthus Annuus and Brassica napus. Water Air Soil Pollut. 2005, 167, 59–71. [Google Scholar] [CrossRef]
- Touiserkani, T.; Haddad, R. Cadmium-induced stress and antioxidative responses in different Brassica napus cultivars. J. Agric. Sci. Technol. 2012, 14, 929–937. [Google Scholar]
- Kim, S.H.; Lee, H.S.; Song, W.Y.; Choi, K.S.; Hur, Y. Chloroplast-targeted BrMT1 (Brassica rapa type-1 metallothionein) enhances resistance to cadmium and ROS in transgenicatabidopsis plants. J. Plant Biol. 2007, 50, 1–7. [Google Scholar] [CrossRef]
- Zhang, M.; Takano, T.; Liu, S.; Zhang, X. Abiotic stress response in yeast and metal-binding ability of a type 2 metallothionein-like protein (PutMT2) from Puccinellia tenuiflora. Mol. Biol. Rep. 2014, 41, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Nagaharu, U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilisation. J. Jpn. Bot. 1935, 7, 389–452. [Google Scholar]
- Von Ruecker, A.A.; Wild, M.; Rao, G.S.; Bidlingmaier, F. Atrial natriuretic peptide protects hepatocytes against damage induced by hypoxia and reactive oxygen. Possible role of intracellular free ionized calcium. J. Clin. Chem. Clin. Biochem. 1989, 27, 531–537. [Google Scholar] [PubMed]
- Palmiter, R.D. The elusive function of metallothioneins. Proc. Natl. Acad. Sci. USA 1998, 95, 8428–8430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tottey, S.; Rondet, S.A.; Borrelly, G.P.; Robinson, P.J.; Rich, P.R.; Robinson, N.J. A copper metallochaperone for photosynthesis and respiration reveals metal-specific targets, interaction with an importer, and alternative sites for copper acquisition. J. Biol. Chem. 2002, 277, 5490–5497. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Philcox, J.; Carey, L.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Xu, D.; Yang, P.; Yu, W.T.; Lv, M.K.; Yuan, D.R.; Yang, Z.H.; Zhang, G.H.; Sun, S.Y.; Wang, X.Q.; et al. A Novel Nonlinear Optical Complex Crystal with an Organic Ligand Coordinated Through an O Atom: Tetrathiocyanatocadmiummercury-Dimethyl Sulfoxide. Cryst. Res. Technol. 2001, 36, 609–614. [Google Scholar] [CrossRef]
- Ansarypour, Z.; Shahpiri, A. Heterologous expression of a rice metallothionein isoform (OsMTI-1b) in Saccharomyces cerevisiae enhances cadmium, hydrogen peroxide and ethanol tolerance. Braz. J. Microbiol. 2017, 48, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Benáková, M.; Ahmadi, H.; Dučaiová, Z.; Tylová, E.; Clemens, S.; Tůma, J. Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Environ. Sci. Pollut. Res. 2017, 24, 20705–20716. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Fan, H.-J.; Ling, H.-Q. Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genom. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Sankoff, D.; Zheng, C.; Zhu, Q. The collapse of gene complement following whole genome duplication. BMC Genom. 2010, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.-H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica Rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Zhao, H.; Fu, F.; Wang, Z.; Zhang, K.; Zhou, Y.; Wang, X.; Wang, R.; Xu, X.; Tang, Z.; et al. Genome-wide survey of flavonoid biosynthesis genes and gene expression analysis between black- and yellow-seeded Brassica napus. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zimeri, A.M.; Dhankher, O.P.; Mccaig, B.; Meagher, R.B. The Plant MT1 Metallothioneins are Stabilized by Binding Cadmiums and are Required for Cadmium Tolerance and Accumulation. Plant Mol. Biol. 2005, 58, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Framond, A.J.D. A metallothionein-like gene from maize (Zea mays) Cloning and characterization. FEBS Lett. 1991, 290, 103–106. [Google Scholar] [CrossRef]
- Zhou, J.; Goldsbrough, P.B. Structure, organization and expression of the metallothionein gene family in Arabidopsis. Mol. Gen. Genet. 1995, 248, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.C.; Singh, K.B. Isolation of a Vicia faba metallothionein-like gene, expression in foliar trichomes. Plant Mol. Biol. 1994, 26, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Shawber, C.; Nofziger, D.; Hsieh, J.J.; Lindsell, C.; Bögler, O.; Hayward, D.; Weinmaster, G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development 1996, 122, 3765–3773. [Google Scholar] [PubMed]
- Liu, J.X.; Zu, Y.G.; Shi, X.G.; Ai, Y.Z.; Du, Y.J.; Fu, Y.J.; An, Z.G. BjMT2, a metallothionein type-2 from Brassica juncea, may effectively remove excess lead from erythrocytes and kidneys of rats. Environ. Toxicol. Pharmacol. 2007, 23, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.O.; Kim, S.H.; Lee, J.; Kim, H.; Lee, H.S.; Kwak, S.S. Three Brassica rapa metallothionein genes are differentially regulated under various stress conditions. Mol. Biol. Rep. 2012, 39, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Gill, R.A.; Ali, B.; Wang, J.; Islam, F.; Ali, S.; Zhou, W. Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 2016, 25, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R. MapChart: Software for the graphical presentation of linkage maps and QTLs. Heredity 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Qu, C.; Fu, F.; Lu, K.; Zhang, K.; Wang, R.; Xu, X.; Wang, M.; Lu, J.; Wan, H.; Zhanglin, T.; et al. Differential accumulation of phenolic compounds and expression of related genes in black-and yellow-seeded Brassica napus. J. Exp. Bot. 2013, 64, 2885–2898. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, Z.; Yuhua, W.; Yinglong, C.; Changming, L. Comparison of Five Endogenous Reference Genes for Specific PCR Detection and Quantification of Brassica napus. J. Agric. Food Chem. 2010, 58, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, T.; He, J.; Chang, W.; Zhang, R.; Liu, M.; Yu, M.; Fan, Y.; Ma, J.; Sun, W. qPrimerDB: A thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Res. 2018, 46, D1229–D1236. [Google Scholar] [CrossRef] [PubMed]

| Groups | Name | Gene ID | Chr. | Start (bp) | End (bp) | Length (bp) | Length (aa) | MW (KDa) | pIs | Exon | Intron |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MT1 | AtMT1 | AT1G07600 | AtChr1 | 2338904 | 2339321 | 138 | 45 | 4.580 | 4.23 | 2 | 1 |
| AtMT1C | AT1G07610 | AtChr1 | 2341542 | 2342123 | 138 | 45 | 4.495 | 4.54 | 2 | 1 | |
| AtMT1B | AT5G56795 | AtChr5 | 22972042 | 22972449 | 156 | 51 | 5.428 | 10.25 | 2 | 1 | |
| BraMT1 | Bra015594 | BraA10 | 766670 | 772706 | 450 | 149 | 16.77 | 9.32 | 4 | 3 | |
| BnaMT1 | BnaA10g04950D | BnaA10 | 2673266 | 2673770 | 138 | 45 | 4.480 | 3.92 | 2 | 1 | |
| BolMT1 | DK501359 | UN | UN | UN | 138 | 45 | 4.412 | 3.92 | UN | UN | |
| BjuMT1 | EF471214 | UN | UN | UN | 138 | 45 | 4.439 | 3.92 | UN | UN | |
| MT2 | AtMT2A | AT3G09390 | AtChr3 | 2889486 | 2890229 | 246 | 81 | 8.163 | 4.35 | 2 | 1 |
| AtMT2B | AT5G02380 | AtChr5 | 506498 | 507244 | 234 | 77 | 7.766 | 4.54 | 2 | 1 | |
| BraMT2A | Bra001309 | BraA03 | 15803598 | 15803933 | 246 | 81 | 8.197 | 4.17 | 2 | 1 | |
| BraMT2B | Bra005720 | BraA03 | 275453 | 275766 | 243 | 80 | 8.033 | 4.29 | 2 | 1 | |
| BraMT2C | Bra009595 | BraA10 | 16182058 | 16182453 | 243 | 80 | 8.031 | 4.29 | 2 | 1 | |
| BraMT2D | Bra028875 | BraA02 | 269835 | 270,238 | 246 | 81 | 8.386 | 4.20 | 2 | 1 | |
| BraMT2E | Bra029765 | BraA05 | 23082833 | 23083178 | 243 | 80 | 8.024 | 4.35 | 2 | 1 | |
| BolMT2A | Bol000591 | Scaffold000521 | 37950 | 38342 | 243 | 80 | 8.031 | 4.29 | 2 | 1 | |
| BolMT2B | Bol011307 | Scaffold000212 | 445183 | 445551 | 243 | 80 | 8.054 | 4.58 | 2 | 1 | |
| BolMT2C | Bol012825 | BolC02 | 305134 | 305536 | 246 | 81 | 8.386 | 4.20 | 2 | 1 | |
| BolMT2D | Bol015273 | BolC03 | 101213 | 101535 | 243 | 80 | 8.137 | 4.29 | 2 | 1 | |
| BolMT2E | Bol023080 | BolC01 | 37533909 | 37534079 | 171 | 56 | 5.920 | 4.15 | 1 | 0 | |
| BolMT2F | Bol033925 | Scaffold000040 | 316614 | 316949 | 246 | 81 | 8.147 | 4.15 | 2 | 1 | |
| BnaMT2A | BnaA03g30680D | BnaA03 | 14857530 | 14858170 | 240 | 79 | 7.966 | 3.81 | 2 | 1 | |
| BnaMT2B | BnaA03g54880D | A03_random | 44751 | 45330 | 243 | 80 | 8.077 | 4.29 | 2 | 1 | |
| BnaMT2C | BnaA05g29010D | BnaA05 | 20416060 | 20416808 | 243 | 80 | 8.024 | 4.35 | 2 | 1 | |
| BnaMT2D | BnaA10g27170D | BnaA10 | 17170773 | 17171571 | 243 | 80 | 8.031 | 4.29 | 2 | 1 | |
| BnaMT2E | BnaAnng00330D | Ann_random | 321245 | 322046 | 246 | 81 | 8.370 | 4.20 | 2 | 1 | |
| BnaMT2F | BnaC02g03550D | BnaC02 | 1685618 | 1686330 | 246 | 81 | 8.386 | 4.20 | 2 | 1 | |
| BnaMT2G | BnaC03g00710D | BnaC03 | 346945 | 347282 | 216 | 71 | 7.284 | 4.08 | 2 | 1 | |
| BnaMT2H | BnaC03g35960D | BnaC03 | 21778025 | 21778740 | 240 | 79 | 7.950 | 3.79 | 2 | 1 | |
| BnaMT2I | BnaC05g43490D | BnaC05 | 40327486 | 40328304 | 243 | 80 | 8.028 | 4.58 | 2 | 1 | |
| BnaMT2J | BnaCnng40400D | Cnn_random | 38972401 | 38973067 | 243 | 80 | 8.031 | 4.29 | 2 | 1 | |
| BjuMT2A | BjuA008858 | BjuA03 | 321697 | 322020 | 243 | 80 | 8.033 | 4.29 | 2 | 1 | |
| BjuMT2B | BjuA020647 | BjuA05 | 29317680 | 29319366 | 312 | 103 | 10.661 | 4.24 | 3 | 2 | |
| BjuMT2C | BjuA040818 | BjuA02 | 430697 | 431097 | 246 | 81 | 8.370 | 4.20 | 2 | 1 | |
| BjuMT2D | BjuA044587 | BjuA10 | 19619066 | 19619458 | 243 | 80 | 8.031 | 4.29 | 2 | 1 | |
| BjuMT2E | BjuB001621 | BjuB08 | 169755 | 170152 | 243 | 80 | 8.059 | 4.29 | 2 | 1 | |
| BjuMT2F | BjuB005939 | BjuB01 | 43381550 | 43383641 | 297 | 98 | 10.057 | 3.93 | 3 | 2 | |
| BjuMT2G | BjuB012072 | BjuB05 | 20387272 | 20387699 | 246 | 81 | 8.353 | 4.11 | 2 | 1 | |
| BjuMT2H | BjuB031838 | BjuB03 | 12766757 | 12767103 | 222 | 73 | 7.596 | 4.35 | 2 | 1 | |
| BjuMT2I | BjuB044439 | BjuB02 | 52757313 | 52757655 | 243 | 80 | 8.061 | 4.29 | 2 | 1 | |
| BniMT2A | BniB001954-PA | BniB08 | 30524166 | 30524483 | 243 | 80 | 8.075 | 4.29 | 2 | 1 | |
| BniMT2B | BniB007929-PA | BniB05 | 2189985 | 2190357 | 243 | 80 | 7.978 | 4.35 | 2 | 1 | |
| BniMT2C | BniB023579-PA | BniB02 | 28213025 | 28213453 | 246 | 81 | 8.353 | 4.11 | 2 | 1 | |
| BniMT2D | BniB039464-PA | BniB07 | 32240383 | 32240733 | 222 | 73 | 7.596 | 4.35 | 2 | 1 | |
| BniMT2E | BniB045064-PA | BniB03 | 44318189 | 44318591 | 225 | 74 | 7.569 | 4.08 | 2 | 1 | |
| MT3 | AtMT3 | AT3G15353 | AtChr3 | 5180642 | 5181586 | 210 | 69 | 7.373 | 4.35 | 3 | 2 |
| BraMT3 | Bra027254 | BraA05 | 20683494 | 20683920 | 204 | 67 | 7.183 | 4.17 | 3 | 2 | |
| BolMT3A | Bol011145 | BolC05 | 28120530 | 28120940 | 204 | 67 | 7.158 | 4.15 | 3 | 2 | |
| BolMT3B | Bol025753 | BolC03 | 17061982 | 17062438 | 198 | 65 | 7.016 | 4.40 | 3 | 2 | |
| BnaMT3A | BnaA05g24200D | BnaA05 | 18177871 | 18178669 | 204 | 67 | 7.127 | 4.15 | 3 | 2 | |
| BnaMT3B | BnaC03g39060D | BnaC03 | 24091592 | 24092154 | 198 | 65 | 7.016 | 4.40 | 3 | 2 | |
| BnaMT3C | BnaC05g38240D | BnaC05 | 37017131 | 37017881 | 204 | 67 | 7.158 | 4.15 | 3 | 2 | |
| BjuMT3 | BjuB025665 | BjuB01 | 40434253 | 40434691 | 204 | 67 | 7.187 | 4.15 | 3 | 2 | |
| BniMT3 | BniB008959-PA | BniB05 | 4957466 | 4957899 | 204 | 67 | 7.187 | 4.15 | 3 | 2 | |
| MT4 | AtMT4B | AT2G23240 | AtChr2 | 9895855 | 9896325 | 261 | 86 | 8.437 | 5.58 | 2 | 1 |
| AtMT4A | AT2G42000 | AtChr2 | 17529243 | 17530443 | 366 | 121 | 12.229 | 7.62 | 4 | 3 | |
| BraMT4 | Bra000590 | BraA03 | 11951235 | 11951571 | 261 | 86 | 8.480 | 7.37 | 2 | 1 | |
| BnaMT4A | BnaA03g23200D | BnaA03 | 11067719 | 11068213 | 261 | 86 | 8.480 | 7.37 | 2 | 1 | |
| BnaMT4B | BnaC03g27400D | BnaC03 | 15895032 | 15895647 | 261 | 86 | 8.468 | 6.97 | 2 | 1 | |
| BjuMT4 | BjuO006263 | Contig407_1_341981 | 122112 | 122451 | 261 | 86 | 8.500 | 6.97 | 2 | 1 | |
| BniMT4 | BniB049568-PA | BniB03 | 29749524 | 29749863 | 261 | 86 | 8.472 | 6.97 | 2 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Zhu, M.; Wang, S.; Ma, G.; Huang, X.; Qiao, C.; Wang, R.; Xu, X.; Liang, Y.; Lu, K.; et al. Genome-Wide Characterization and Analysis of Metallothionein Family Genes That Function in Metal Stress Tolerance in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2181. https://doi.org/10.3390/ijms19082181
Pan Y, Zhu M, Wang S, Ma G, Huang X, Qiao C, Wang R, Xu X, Liang Y, Lu K, et al. Genome-Wide Characterization and Analysis of Metallothionein Family Genes That Function in Metal Stress Tolerance in Brassica napus L. International Journal of Molecular Sciences. 2018; 19(8):2181. https://doi.org/10.3390/ijms19082181
Chicago/Turabian StylePan, Yu, Meichen Zhu, Shuxian Wang, Guoqiang Ma, Xiaohu Huang, Cailin Qiao, Rui Wang, Xinfu Xu, Ying Liang, Kun Lu, and et al. 2018. "Genome-Wide Characterization and Analysis of Metallothionein Family Genes That Function in Metal Stress Tolerance in Brassica napus L." International Journal of Molecular Sciences 19, no. 8: 2181. https://doi.org/10.3390/ijms19082181






