Differentiation of Adsorptive and Viscous Effects of Dietary Fibres on Bile Acid Release by Means of In Vitro Digestion and Dialysis
Abstract
:1. Introduction
2. Results
2.1. Fibre Composition
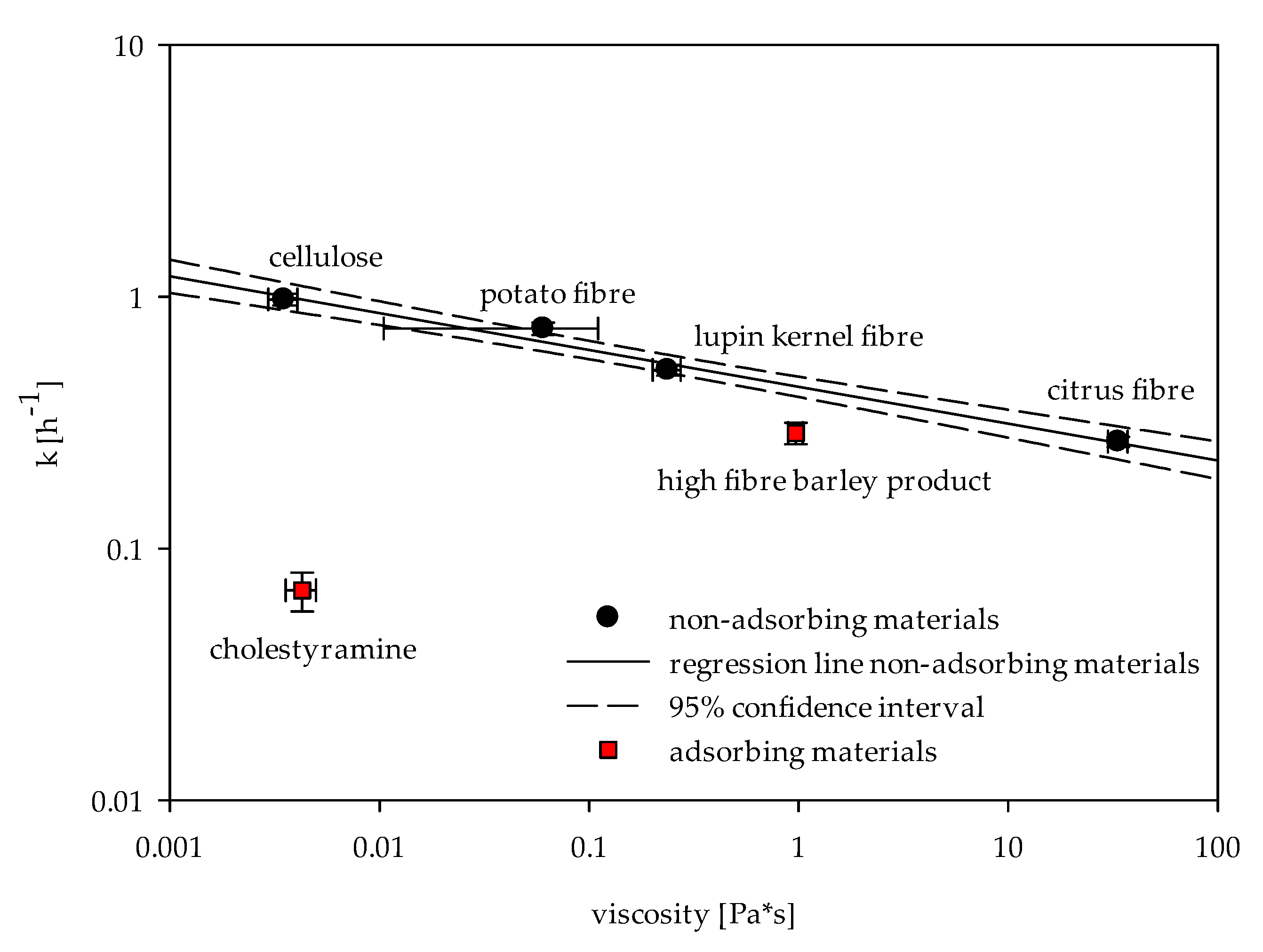
2.2. Viscosity
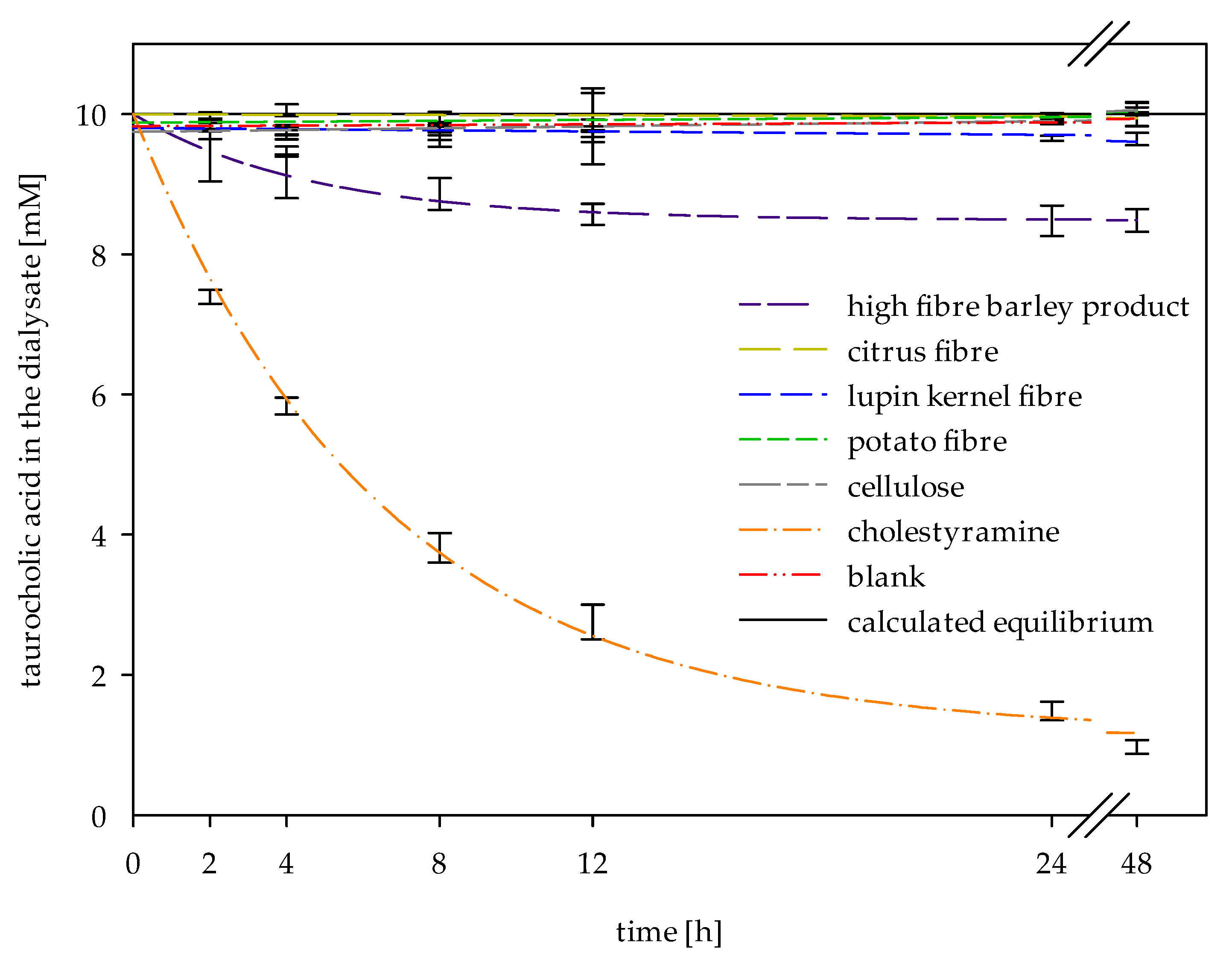
2.3. In Vitro Determinations of Bile Acid Reabsorption
2.4. Determination of Adsorptive Effects on Bile Acids
3. Discussion
3.1. In Vitro Methodologies
3.2. Evaluation of Dietary Fibre-Rich Materials
4. Materials and Methods
4.1. Chemicals and Enzyme Preparations
4.2. Dietary Fibre-Rich Materials
4.3. Fibre Composition
4.4. Viscosity
4.5. In Vitro Determination of Bile Acid Reabsorption
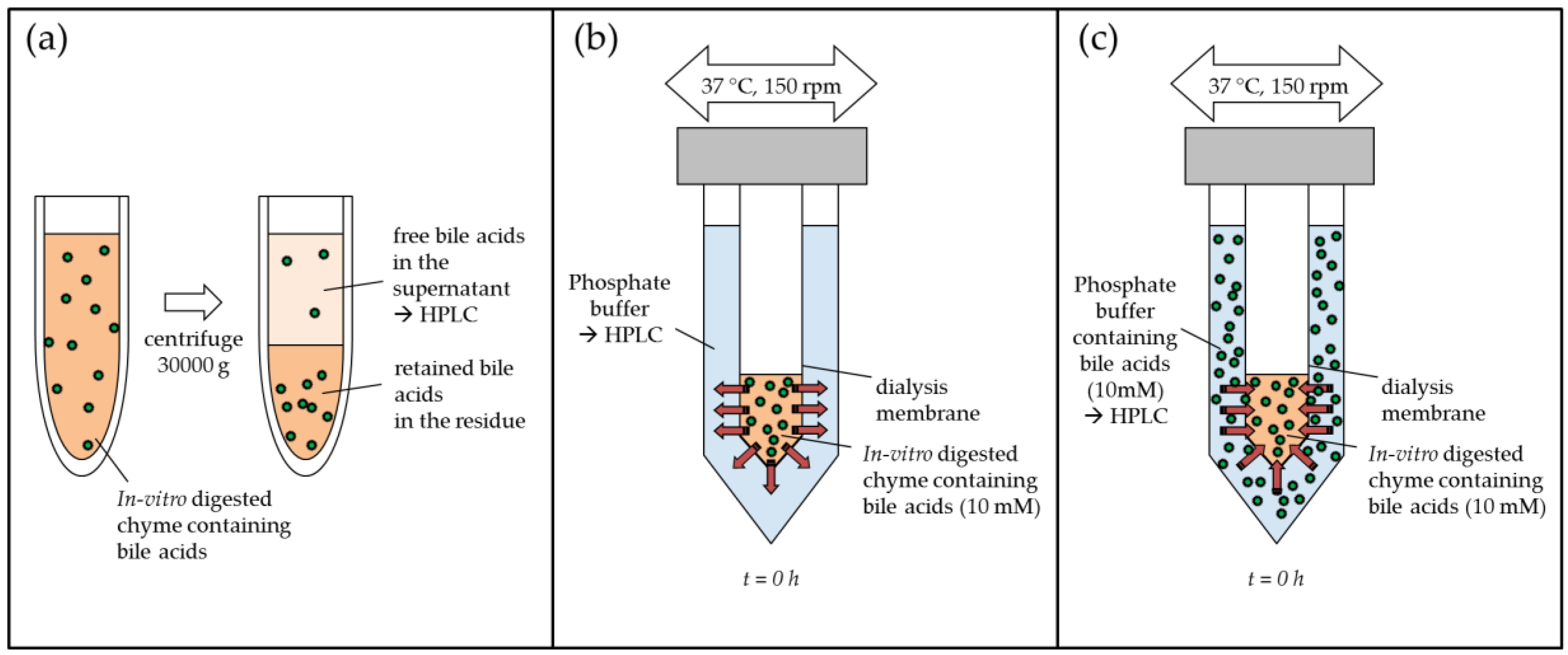
4.5.1. In Vitro Digestion
4.5.2. Bile Acid Binding with Centrifugation Method
4.5.3. Bile Acid Release from Simulated Chyme Using Dialysis
4.6. Inverse Dialysis Model for the Determination of Adsorptive Effects on Bile Acids
4.7. Bile Acid Quantification Using HPLC
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | dry matter |
| HPLC | high-performance liquid chromatography |
| IDF | insoluble dietary fibre |
| SDF | soluble dietary fibre |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
| SSF | simulated salivary fluid |
| TDF | total dietary fibre |
References
- Stamler, J.; Daviglus, M.L.; Garside, D.B.; Dyer, A.R.; Greenland, P.; Neaton, J.D. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000, 284, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D. Dietary Fibres and Dietary Lipids. In Advanced Dietary Fibre Technology; McCleary, B.V., Prosky, L., Eds.; Blackwell Science: Malden, MA, USA, 2001; pp. 177–185. ISBN 9780632056347. [Google Scholar]
- Würsch, P.; Pi-Sunyer, F.X. The Role of Viscous Soluble Fiber in the Metabolic Control of Diabetes: A review with special emphasis on cereals rich in β-glucan. Diabetes Care 1997, 20, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, A.T.; Lichtenstein, A.H. Fiber and Cardiovascular Disease Risk: How Strong Is the Evidence? J. Cardiovasc. Nurs. 2006, 21, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lia, A.; Hallmans, G.; Sandberg, A.S.; Sundberg, B.; Aman, P.; Andersson, H. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am. J. Clin. Nutr. 1995, 62, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Borum, M.L.; Shehan, K.L.; Fromm, H.; Jahangeer, S.; Floor, M.K.; Alabaster, O. Fecal bile acid excretion and composition in response to changes in dietary wheat bran, fat and calcium in the rat. Lipids 1992, 27, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diez, F.; Garcia-Mediavilla, V. Pectin feeding influences fecal bile acid excretion, hepatic bile acid and cholesterol synthesis and serum cholesterol in rats. J. Nutr. 1996, 126, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Dongowski, G. Interactions between dietary fibre-rich preparations and glycoconjugated bile acids in vitro. Food Chem. 2007, 104, 390–397. [Google Scholar] [CrossRef]
- Drzikova, B.; Dongowski, G.; Gebhardt, E. Dietary fibre-rich oat-based products affect serum lipids, microbiota, formation of short-chain fatty acids and steroids in rats. Br. J. Nutr. 2005, 94, 1012–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlon, T.S.; Chow, F.I. In Vitro Binding of Bile Acids by Rice Bran, Oat Bran, Wheat Bran, and Corn Bran. Cereal Chem. 2000, 77, 518–521. [Google Scholar] [CrossRef]
- Adiotomre, J.; Eastwood, M.A.; Edwards, C.A.; Brydon, W.G. Dietary fiber: In vitro methods that anticipate nutrition and metabolic activity in humans. Am. J. Clin. Nutr. 1990, 52, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Flanagan, B.M.; Shelat, K.; Gilbert, R.G.; Gidley, M.J. Kinetic analysis of bile salt passage across a dialysis membrane in the presence of cereal soluble dietary fibre polymers. Food Chem. 2012, 134, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Zacherl, C.; Eisner, P.; Engel, K.-H. In vitro model to correlate viscosity and bile acid-binding capacity of digested water-soluble and insoluble dietary fibres. Food Chem. 2011, 126, 423–428. [Google Scholar] [CrossRef]
- Cornfine, C.; Hasenkopf, K.; Eisner, P.; Schweiggert, U. Influence of chemical and physical modification on the bile acid binding capacity of dietary fibre from lupins (Lupinus angustifolius L.). Food Chem. 2010, 122, 638–644. [Google Scholar] [CrossRef]
- Macheras, P.; Koupparis, M.; Tsaprounis, C. An automated flow injection-serial dynamic dialysis technique for drug-protein binding studies. Int. J. Pharm. 1986, 30, 123–132. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Chapman, M.H.; Smith, G.E. In vitro binding of bile acids by okra, beets, asparagus, eggplant, turnips, green beans, carrots, and cauliflower. Food Chem. 2007, 103, 676–680. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Optimizing the Molecular Weight of Oat β-Glucan for in Vitro Bile Acid Binding and Fermentation. J. Agric. Food Chem. 2011, 59, 10322–10328. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, T.S.; Woodruff, C.L. In Vitro Binding of Bile Acids by Rice Bran, Oat Bran, Barley and β-Glucan Enriched Barley. Cereal Chem. 2003, 80, 260–263. [Google Scholar] [CrossRef]
- Oakenfull, D.G.; Fenwick, D.E. Adsorption of bile salts from aqueous solution by plant fibre and cholestyramine. Br. J. Nutr. 1978, 40, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- van Bennekum, A.M.; Nguyen, D.V.; Schulthess, G.; Hauser, H.; Phillips, M.C. Mechanisms of cholesterol-lowering effects of dietary insoluble fibres: Relationships with intestinal and hepatic cholesterol parameters. Br. J. Nutr. 2005, 94, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Dikeman, C.L.; Fahey, G.C. Viscosity as Related to Dietary Fiber: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Levrat-Verny, M.-A.; Behr, S.; Mustad, V.; Rémésy, C.; Demigné, C. Low Levels of Viscous Hydrocolloids Lower Plasma Cholesterol in Rats Primarily by Impairing Cholesterol Absorption. J. Nutr. 2000, 130, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Dongowski, G.; Huth, M.; Gebhardt, E. Steroids in the intestinal tract of rats are affected by dietary-fibre-rich barley-based diets. Br. J. Nutr. 2003, 90, 895–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Piva, M.; Labuza, T.P. Evaluation of Water Binding Capacity (WBC) of Food Fiber Sources. J. Food Sci. 1984, 49, 59–63. [Google Scholar] [CrossRef]
- Lærke, H.N.; Meyer, A.S.; Kaack, K.V.; Larsen, T. Soluble fiber extracted from potato pulp is highly fermentable but has no effect on risk markers of diabetes and cardiovascular disease in Goto-Kakizaki rats. Nutr. Res. 2007, 27, 152–160. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.M.; Truswell, A.S. Effect of citrus pectin on blood lipids and fecal steroid excretion in man. Am. J. Clin. Nutr. 1977, 30, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosaeus, I.; Carlsson, N.G.; Sandberg, A.S.; Andersson, H. Effect of wheat bran and pectin on bile acid and cholesterol excretion in ileostomy patients. Hum. Nutr. Clin. Nutr. 1986, 40, 429–440. [Google Scholar] [PubMed]
- Chau, C.F.; Huang, Y.L.; Lin, C.Y. Investigation of the cholesterol-lowering action of insoluble fibre derived from the peel of Citrus sinensis L. cv. Liucheng. Food Chem. 2004, 87, 361–366. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to barley beta-glucans and lowering of blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2470–2484. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; White, P.J. In Vitro Bile-Acid Binding and Fermentation of High, Medium, and Low Molecular Weight β-Glucan. J. Agric. Food Chem. 2010, 58, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Sayar, S.; Jannink, J.-L.; White, P.J. In Vitro Bile Acid Binding of Flours from Oat Lines Varying in Percentage and Molecular Weight Distribution of β-Glucan. J. Agric. Food Chem. 2005, 53, 8797–8803. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Flanagan, B.M.; Gidley, M.J. Molecular interactions between cereal soluble dietary fibre polymers and a model bile salt deduced from 13C NMR titration. J. Cereal Sci. 2010, 52, 444–449. [Google Scholar] [CrossRef]
- Bowles, R.K.; Morgan, K.R.; Furneaux, R.H.; Coles, G.D. 13C CP/MAS NMR study of the interaction of bile acids with barley β-d-glucan. Carbohydr. Polym. 1996, 29, 7–10. [Google Scholar] [CrossRef]
- Oscarsson, M.; Andersson, R.; Salomonsson, A.C.; Åman, P. Chemical Composition of Barley Samples Focusing on Dietary Fibre Components. J. Cereal Sci. 1996, 24, 161–170. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Woodruff, C.L. In vitro binding of bile acids by soy protein, pinto beans, black beans and wheat gluten. Food Chem. 2002, 79, 425–429. [Google Scholar] [CrossRef]
- Araki, Y.; Andoh, A.; Fujiyama, Y.; Kanauchi, O.; Takenaka, K.; Higuchi, A.; Bamba, T. Germinated Barley Foodstuff Exhibits Different Adsorption Properties for Hydrophilic versus Hydrophobic Bile Acids. Digestion 2001, 64, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, C.M.; Baxter, A.L.; Johnson, S.K. Water-binding capacity and viscosity of Australian sweet lupin kernel fibre under in vitro conditions simulating the human upper gastrointestinal tract. Int. J. Food Sci. Nutr. 2005, 56, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fechner, A.; Kiehntopf, M.; Jahreis, G. The Formation of Short-Chain Fatty Acids Is Positively Associated with the Blood Lipid–Lowering Effect of Lupin Kernel Fiber in Moderately Hypercholesterolemic Adults. J. Nutr. 2014, 144, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostina, A.; Antonioni, C.; Resta, D.; Arnoldi, A.; Bez, J.; Knauf, U.; Wäsche, A. Optimization of a Pilot-Scale Process for Producing Lupin Protein Isolates with Valuable Technological Properties and Minimum Thermal Damage. J. Agric. Food Chem. 2006, 54, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 20th ed.; The Scientific Association Dedicated to Analytical Excellence: Washington, DC, USA, 2016; Volume 2, ISBN 978-0935584875. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; ISBN 3-900051-07-0. [Google Scholar]


| Sample | TDF (g/100 g DM) | IDF (g/100 g DM) | SDF (g/100 g DM) | SDF/TDF (%) |
|---|---|---|---|---|
| High-fibre barley product | 29.2 ± 1.5 a | 9.5 ± 0.2 a | 19.8 ± 1.5 e | 67.3 ± 1.0 e |
| Citrus fibre | 91.2 ± 0.0 d | 79.8 ± 1.2 d | 14.9 ± 0.3 d | 15.7 ± 0.3 c |
| Lupin kernel fibre | 83.4 ± 0.7 c | 78.6 ± 0.4 c | 4.8 ± 0.6 b | 5.8 ± 0.7 b |
| Potato fibre | 67.8 ± 1.9 b | 56.5 ± 0.8 b | 11.2 ± 1.7 c | 16.5 ± 2.2 d |
| Cellulose | 100.0 ± 0.5 e | 99.1 ± 0.2 e | 0.8 ± 0.4 a | 0.8 ± 0.4 a |
| Sample | R2 | Cf (mM) | k (h−1) |
|---|---|---|---|
| High-fibre barley product | 0.96 | 0.86 ± 0.02 b | 0.29 ± 0.03 a,b |
| Citrus fibre | 0.99 | 0.99 ± 0.01 c | 0.27 ± 0.01 a,b |
| Lupin kernel fibre | 0.99 | 0.96 ± 0.01 c | 0.51 ± 0.03 b,c |
| Potato fibre | 0.99 | 1.00 ± 0.01 c | 0.75 ± 0.04 c,d |
| Cellulose | 1.00 | 1.01 ± 0.01 c | 0.98 ± 0.05 d |
| Cholestyramine | 0.92 | 0.09 ± 0.01 a | 0.07 ± 0.01 a |
| Blank | 1.00 | 1.00 ± 0.00 c | 1.45 ± 0.07 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumann, S.; Schweiggert-Weisz, U.; Bader-Mittermaier, S.; Haller, D.; Eisner, P. Differentiation of Adsorptive and Viscous Effects of Dietary Fibres on Bile Acid Release by Means of In Vitro Digestion and Dialysis. Int. J. Mol. Sci. 2018, 19, 2193. https://doi.org/10.3390/ijms19082193
Naumann S, Schweiggert-Weisz U, Bader-Mittermaier S, Haller D, Eisner P. Differentiation of Adsorptive and Viscous Effects of Dietary Fibres on Bile Acid Release by Means of In Vitro Digestion and Dialysis. International Journal of Molecular Sciences. 2018; 19(8):2193. https://doi.org/10.3390/ijms19082193
Chicago/Turabian StyleNaumann, Susanne, Ute Schweiggert-Weisz, Stephanie Bader-Mittermaier, Dirk Haller, and Peter Eisner. 2018. "Differentiation of Adsorptive and Viscous Effects of Dietary Fibres on Bile Acid Release by Means of In Vitro Digestion and Dialysis" International Journal of Molecular Sciences 19, no. 8: 2193. https://doi.org/10.3390/ijms19082193







