Chemopreventive Effects of Phytochemicals and Medicines on M1/M2 Polarized Macrophage Role in Inflammation-Related Diseases
Abstract
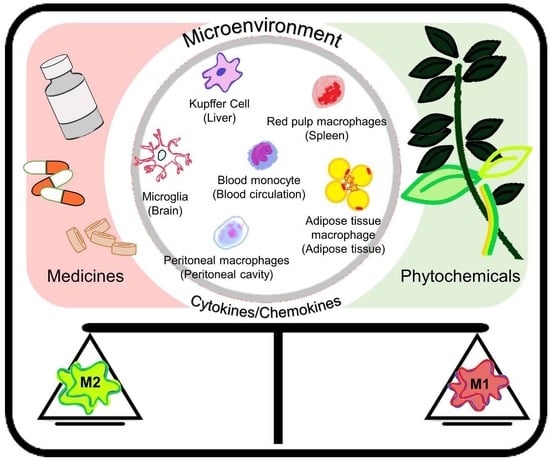
:1. Introduction
2. Preventive and Therapeutic Strategies Dealing with M1/M2 Macrophages Polarization
3. Role of Macrophages in Intestinal and Colorectal Disease
4. Role of Macrophages in Liver Protection
5. Role of Macrophages in Brain Injuries and Neuron Protection
6. Role of Macrophages in Obesity and Metabolic Diseases
7. Role of Macrophages in Joint Diseases and Hemodynamic Disease
8. Role of Macrophages in Cancer Progression
9. Conclusions
Funding
Conflicts of Interest
References
- Ohashi, W.; Hattori, K.; Hattori, Y. Control of Macrophage Dynamics as a Potential Therapeutic Approach for Clinical Disorders Involving Chronic Inflammation. J. Pharmacol. Exp. Ther. 2015, 354, 240–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreesen, R.; Gadd, S.; Brugger, W.; Löhr, G.W.; Atkins, R.C. Activation of Human Monocyte-Derived Macrophages Cultured on Teflon: Response to Interferon-γ during Terminal Maturation in vitro. Immunobiology 1988, 177, 186–198. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428. [Google Scholar] [CrossRef] [PubMed]
- Labonte, A.C.; Tosello-Trampont, A.-C.; Hahn, Y.S. The Role of Macrophage Polarization in Infectious and Inflammatory Diseases. Mol. Cells 2014, 37, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.M.; Liu, J.C.; Trujillo-de Santiago, G.; Cha, B.-H.; Vishwakarma, A.; Ghaemmaghami, A.M.; Khademhosseini, A. Delivery strategies to control inflammatory response: Modulating M1–M2 polarization in tissue engineering applications. J. Controll. Release 2016, 240, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Peng, M.; Yang, Z.; Zhou, X.; Deng, Y.; Jiang, C.; Xiao, M.; Wang, J. 3D-printed IFN-γ-loading calcium silicate-β-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomater. 2018, 71, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Russo, V.; Di Marcantonio, L.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mattioli, M.; Barboni, B. M1 and M2 macrophage recruitment during tendon regeneration induced by amniotic epithelial cell allotransplantation in ovine. Res. Vet. Sci. 2016, 105, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Editorial board of Nature Immunology. A current view on inflammation. Nat. Immunol. 2017, 18, 825. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Vecchi, A.; Allavena, P. Pharmacological modulation of monocytes and macrophages. Curr. Opin. Pharmacol. 2014, 17, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Transl. 2017, 10, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Jou, I.M.; Lin, C.-F.; Tsai, K.-J.; Wei, S.-J. Macrophage-Mediated Inflammatory Disorders. Mediat. Inflamm. 2013, 2013, 316482. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Zhao, Y.; Zhu, Q.; Zhou, Y.; Zhao, Y.; Zhang, T.; Guo, Q.; Lu, N. LZ205, a newly synthesized flavonoid compound, exerts anti-inflammatory effect by inhibiting M1 macrophage polarization through regulating PI3K/AKT/mTOR signaling pathway. Exp. Cell Res. 2018, 364, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Camille, N.; Dealtry, G. Regulation of M1/M2 macrophage polarization by Sutherlandia frutescens via NFkB and MAPK signaling pathways. S. Afr. J. Bot. 2018, 116, 42–51. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Zhou, J.; Guo, N.; Ma, W.-G.; Huang, X.; Wang, H.; Yuan, Z.-Y. Curcumin retunes cholesterol transport homeostasis and inflammation response in M1 macrophage to prevent atherosclerosis. Biochem. Biophys. Res. Commun. 2015, 467, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Hisamatsu, T.; Suzuki, H.; Mori, K.; Kitazume, M.T.; Shimamura, K.; Mizuno, S.; Nakamoto, N.; Matsuoka, K.; Naganuma, M.; et al. Glycolysis regulates LPS-induced cytokine production in M2 polarized human macrophages. Immunol. Lett. 2017, 183, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Climaco-Arvizu, S.; Domínguez-Acosta, O.; Cabañas-Cortés, M.A.; Rodríguez-Sosa, M.; Gonzalez, F.J.; Vega, L.; Elizondo, G. Aryl hydrocarbon receptor influences nitric oxide and arginine production and alters M1/M2 macrophage polarization. Life Sci. 2016, 155, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Gong, Y.; Meng, W.; Yuan, M.; Zhu, H.; Ying, M.; He, Q.; Cao, J.; Yang, B. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemopreventive effects on colon cancer. Cancer Lett. 2017, 388, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, Y.; Zhang, Y.; Tian, J.; Li, J.; Li, Z.; He, Z.; Chai, R.; Liu, F.; Zhang, T.; et al. Irgm1 promotes M1 but not M2 macrophage polarization in atherosclerosis pathogenesis and development. Atherosclerosis 2016, 251, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Song, P.; Zhou, H.; Li, A.; Ma, Y.; Zhang, X.; Liu, H.; Xu, G.; Zhou, Y.; Wu, X.; et al. Pentamethoxyflavanone regulates macrophage polarization and ameliorates sepsis in mice. Biochem. Pharmacol. 2014, 89, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Yu, W.; Huang, Y.; Chen, J.; Fu, H.; et al. Activation of PPARγ by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y.; et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-M.; Kang, G.-D.; Van Le, T.K.; Lim, S.-M.; Jang, D.-S.; Kim, D.-H. 4-Methoxylonchocarpin attenuates inflammation by inhibiting lipopolysaccharide binding to Toll-like receptor of macrophages and M1 macrophage polarization. Int. Immunopharmacol. 2017, 45, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, J.; Shi, Q.; Xiao, Y.; Wang, W.; Chen, G.; Zhao, Z.; Wang, R.; Xiao, H.; Hou, C.; et al. Tim-3 promotes intestinal homeostasis in DSS colitis by inhibiting M1 polarization of macrophages. Clin. Immunol. 2015, 160, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, S.-J.; Lee, A.-Y.; Huang, J.; Chung, H.-Y.; Im, D.-S. Ginsenoside Rg3 promotes inflammation resolution through M2 macrophage polarization. J. Ginseng Res. 2018, 42, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-J.; Lo, C.-Y.; Wang, B.-J.; Chiou, R.Y.-Y.; Lin, S.-M. Theaflavin-3, 3′-digallate, a black tea polyphenol, attenuates adipocyte-activated inflammatory response of macrophage associated with the switch of M1/M2-like phenotype. J. Funct. Foods 2014, 11, 36–48. [Google Scholar] [CrossRef]
- Lu, H.; Bowler, N.; Harshyne, L.A.; Craig Hooper, D.; Krishn, S.R.; Kurtoglu, S.; Fedele, C.; Liu, Q.; Tang, H.-Y.; Kossenkov, A.V.; et al. Exosomal αvβ6 integrin is required for monocyte M2 polarization in prostate cancer. Matrix Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Meireles, M.; Marques, C.; Norberto, S.; Santos, P.; Fernandes, I.; Mateus, N.; Faria, A.; Calhau, C. Anthocyanin effects on microglia M1/M2 phenotype: Consequence on neuronal fractalkine expression. Behav. Brain Res. 2016, 305, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Wang, S.; Li, Q. Sevoflurane suppresses microglial M2 polarization. Neurosci. Lett. 2017, 655, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Kim, H.-J.; Na, H.; Nam, M.-W.; Kim, J.-Y.; Kim, J.-S.; Koo, S.-H.; Lee, M.-O. RORα Induces KLF4-Mediated M2 Polarization in the Liver Macrophages that Protect against Nonalcoholic Steatohepatitis. Cell Rep. 2017, 20, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.-F.; Gao, C.-C.; Yi, J.; Zhao, J.-L.; Liang, S.-Q.; Zhao, Y.; Ye, Y.-C.; Bai, J.; Zheng, Q.-J.; Dou, K.-F.; et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J. Hepatol. 2017, 67, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Klüter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017, 222, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zheng, K.; Su, Z.; Su, H.; Zhong, M.; He, X.; Zhou, C.; Chen, H.; Xiong, Q.; Zhang, Y. Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage. J. Neuroimmunol. 2016, 299, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Yi, H.; Xu, L.; Zhang, Z. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience 2015, 294, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Feng, L.; Song, P.; Xu, F.; Li, A.; Wang, Y.; Shen, Y.; Wu, X.; Luo, Q.; Wu, X.; et al. Isomeranzin suppresses inflammation by inhibiting M1 macrophage polarization through the NF-κB and ERK pathway. Int. Immunopharmacol. 2016, 38, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, F.; Yang, H.; Li, R.; Guo, H.; Hu, L. Diosgenin glucoside provides neuroprotection by regulating microglial M1 polarization. Int. Immunopharmacol. 2017, 50, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, F.; Liu, L.; Feng, L.; Wu, X.; Shen, Y.; Sun, Y.; Wu, X.; Xu, Q. (+)-Borneol improves the efficacy of edaravone against DSS-induced colitis by promoting M2 macrophages polarization via JAK2-STAT3 signaling pathway. Int. Immunopharmacol. 2017, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hao, H.; Xie, Z.; Cheng, Y.; Yin, Y.; Xie, M.; Huang, H.; Gao, J.; Liu, H.; Tong, C.; et al. M2 macrophages infusion ameliorates obesity and insulin resistance by remodeling inflammatory/macrophages’ homeostasis in obese mice. Mol. Cell. Endocrinol. 2017, 443, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Chen, J.; Chen, T.; Shi, Z.; Lei, M.; Zhang, Y.; Bai, P.; Li, Y.; Fei, X. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int. Immunopharmacol. 2016, 30, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jin, Z.; Yu, J.; Liang, J.; Yang, Q.; Li, F.; Shi, X.; Zhu, X.; Zhang, X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 2016, 35, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A. Temporal profile of M1 and M2 responses in the hippocampus following early 24h of neurotrauma. J. Neurol. Sci. 2015, 357, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int. Immunopharmacol. 2017, 51, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Qin, H.; Shi, Q.; Zhang, Y.; Zhou, F.; Wu, H.; Ding, S.; Niu, Z.; Lu, Y.; Shen, P. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem. Pharmacol. 2014, 89, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Wijesundera, K.K.; Izawa, T.; Tennakoon, A.H.; Golbar, H.M.; Tanaka, M.; Kuwamura, M.; Yamate, J. M1-/M2-macrophage polarization in pseudolobules consisting of adipohilin-rich hepatocytes in thioacetamide (TAA)-induced rat hepatic cirrhosis. Exp. Mol. Pathol. 2016, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.N.; Rodrigues, M.A.; Gomes, D.A.; Salgado, B.S.; Cassali, G.D. Tumour-associated macrophages: Relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours. Veter. J. 2018, 234, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, L.; Schutgens, R.E.G.; Coeleveld, K.; Mastbergen, S.C.; Roosendaal, G.; Biesma, D.H.; Lafeber, F.P.J.G. Hemarthrosis in hemophilic mice results in alterations in M1-M2 monocyte/macrophage polarization. Thromb. Res. 2014, 133, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Geil Nickell, C.R.; Chen, K.Y.; McClain, J.A.; Nixon, K. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol 2017, 62, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Vomhof-DeKrey, E.; Darland, D.; Ghribi, O.; Bundy, A.; Roemmich, J.; Claycombe, K. Maternal low protein diet leads to placental angiogenic compensation via dysregulated M1/M2 macrophages and TNFα expression in Sprague-Dawley rats. J. Reprod. Immunol. 2016, 118, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, M.; Li, N.; Li, Z.; Li, H.; Shao, S.; Zou, K.; Zou, L. β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem. Biophys. Res. Commun. 2017, 490, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.P.; Minogue, A.M.; Falvey, A.; Lynch, M.A. Involvement of IGF-1 and Akt in M1/M2 activation state in bone marrow-derived macrophages. Exp. Cell Res. 2015, 335, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Sharma, P.K.; Mukhopadhaya, A. Vibrio cholerae porin OmpU mediates M1-polarization of macrophages/monocytes via TLR1/TLR2 activation. Immunobiology 2015, 220, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Wang, Y.; Zhang, L.; Wei, J.; Zhang, X.; He, F.; Zhang, L. Casein Kinase 2 Interacting Protein-1 regulates M1 and M2 inflammatory macrophage polarization. Cell. Signal. 2017, 33, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.; Saima, F.T.; Rhee, K.-J.; Hwang, S.; Kim, M.Y.; Baik, S.K.; Eom, Y.W.; Kim, H.-S. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem. Biophys. Res. Commun. 2018, 498, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Neog, M.K.; Sultana, F.; Rasool, M. Targeting RAW 264.7 macrophages (M1 type) with Withaferin-A decorated mannosylated liposomes induces repolarization via downregulation of NF-κB and controlled elevation of STAT-3. Int. Immunopharmacol. 2018, 61, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, Q.; Li, S.; Meng, F.; Li, X.; Gong, X. miR-330-5p/Tim-3 axis regulates macrophage M2 polarization and insulin resistance in diabetes mice. Mol. Immunol. 2018, 95, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, M.A.R.; Wunderlich, F.T. Macrophage function in obesity-induced inflammation and insulin resistance. Pflug. Arch. 2017, 469, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.; Saikia, P.; McMullen, M.; McCullough, R.; Nagy, L. SAT-459—miRNA regulation of macrophage M1/M2 polarization of Kupffer cells and PBMCs in alcoholic liver disease. J. Hepatol. 2018, 68, S813. [Google Scholar] [CrossRef]
- White, E.S.; Mantovani, A.R. Inflammation, wound repair, and fibrosis: Reassessing the spectrum of tissue injury and resolution. J. Pathol. 2013, 229, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, L.; Liu, L.; Ruan, Q.; Zhang, X.; Hong, W.; Wu, S.; Jin, G.; Bai, Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 2018, 154, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiong, M.; Chen, C.; Du, L.; Liu, Z.; Shi, Y.; Zhang, M.; Gong, J.; Song, X.; Xiang, R.; et al. Legumain, an asparaginyl endopeptidase, mediates the effect of M2 macrophages on attenuating renal interstitial fibrosis in obstructive nephropathy. Kidney Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Vivar, R.; Boza, P.; Muñoz, C.; Bolivar, S.; Anfossi, R.; Osorio, J.M.; Olivares-Silva, F.; García, L.; Díaz-Araya, G. Cardiac fibroblast cytokine profiles induced by proinflammatory or profibrotic stimuli promote monocyte recruitment and modulate macrophage M1/M2 balance in vitro. J. Mol. Cell. Cardiol. 2016, 101, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wijesundera, K.K.; Izawa, T.; Tennakoon, A.H.; Golbar, H.M.; Tanaka, M.; Kuwamura, M.; Yamate, J. M1-/M2-macrophages contribute to the development of GST-P-positive preneoplastic lesions in chemically-induced rat cirrhosis. Exp. Toxicol. Pathol. 2015, 67, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Ciotti, G.M.P.; Braun, D.; Kalinin, S.; Currò, D.; Dello Russo, C.; Coli, A.; Mangiola, A.; Anile, C.; Feinstein, D.L.; et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci. Lett. 2017, 645, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Shi, M.; Zheng, C.; Shen, D.; Zhu, J.; Zheng, X.; Cui, L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018, 318, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Wu, Q.; Yuan, W.; Chen, F.; Du, D. Role of microglia M1/M2 polarisation in the paraventricular nucleus: New insight into the development of stress-induced hypertension in rats. Auton. Neurosci. [CrossRef] [PubMed]
- Wang, J.; Xing, H.; Wan, L.; Jiang, X.; Wang, C.; Wu, Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed. Pharmacother. 2018, 105, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Moehle, M.S.; West, A.B. M1 and M2 immune activation in Parkinson’s Disease: Foe and ally? Neuroscience 2015, 302, 59–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Sun, Z.; Jin, M.; Tu, Y.; Wang, S.; Yang, X.; Chen, Q.; Zhang, X.; Han, Y.; Pi, R. Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non-specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF-κB pathway. J. Neuroimmunol. 2017, 305, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.-Y.; Zhang, S.; Gao, Y.; Wang, Z.-Z.; Chen, N.-H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharmacol. 2015, 25, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Lu, H.; Yang, Q.; Wu, H.; Wang, J. Microglial Polarization and Inflammatory Mediators After Intracerebral Hemorrhage. Mol. Neurobiol. 2017, 54, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Han, X.; Li, Q.; Yang, Q.-W.; Wang, J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 2017, 13, 420–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Barrett, J.P.; Alvarez-Croda, D.-M.; Stoica, B.A.; Faden, A.I.; Loane, D.J. NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 2016, 58, 291–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Li, L.; Jiang, B.; Feng, Z.; Yang, L.; Tang, J.; Chen, Q.; Zhang, J.; Tan, Q.; Feng, H.; et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav. Immun. 2016, 58, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Ye, J.; Han, Y.; Huang, L.; Yang, H.; Jiang, W.; Chen, S.; Zhong, W.; Zeng, H.; Li, D.Y. Hypertonic saline regulates microglial M2 polarization via miR-200b/KLF4 in cerebral edema treatment. Biochem. Biophys. Res. Commun. 2018, 499, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Campos, B.; Hart, K.; Thakar, C.; Adeoye, O. M2 Monocyte Microparticles Are Increased in Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2017, 26, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wang, L.; Li, F.; Yang, M.; Song, L.; Tian, F.; Yukht, A.; Shah, P.K.; Rothenberg, M.E.; Sharifi, B.G. Oxidized LDL activated eosinophil polarize macrophage phenotype from M2 to M1 through activation of CD36 scavenger receptor. Atherosclerosis 2017, 263, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Takagi, Y.; Kawamoto, E.; Park, E.J.; Usuda, H.; Wada, K.; Shimaoka, M. Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor γ expression. Exp. Cell Res. 2018, 367, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Rosshirt, N.; Engbarth, T.; Gotterbarm, T.; Hagmann, S.; Moradi, B. M1/M2 macrophages induce chondral MMP/ADAMTS enzyme secretion in a direct co-culture experiment. Osteoarthr. Cartil. 2018, 26, S129. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas Subhra, K.; Galdiero Maria, R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2012, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Juusola, M.; Mustonen, H.; Vainionpää, S.; Vähä-Koskela, M.; Puolakkainen, P.; Seppänen, H. The effect of pancreatic cancer patient derived serum on macrophage M1/M2 polarization. Pancreatology 2018, 18, S147–S148. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.-X.; Qiao, S.-L.; An, H.-W.; Ma, Y.; Qiao, Z.-Y.; Rajapaksha, R.P.Y.J.; Wang, H. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials 2017, 112, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Asif, M.; Singh, V.; Dubey, P.; Ahmad Malik, S.; Lone M-U, D.; Tewari, B.N.; Baghel, K.S.; Pal, S.; Nagar, G.K.; et al. M2 polarization of macrophages by Oncostatin M in hypoxic tumor microenvironment is mediated by mTORC2 and promotes tumor growth and metastasis. Cytokine 2018. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L. Melanoma exosome induction of endothelial cell GM-CSF in pre-metastatic lymph nodes may result in different M1 and M2 macrophage mediated angiogenic processes. Med. Hypotheses 2016, 94, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, J.; Zeng, X.; Zhao, L.; Wei, Q.; Yu, L.; Wang, X.; Yu, Z.; Cao, Y.; Shan, F.; Wei, M. miR-181a Induces Macrophage Polarized to M2 Phenotype and Promotes M2 Macrophage-mediated Tumor Cell Metastasis by Targeting KLF6 and C/EBPα. Mol. Ther. Nucleic Acids 2016, 5, e368. [Google Scholar] [CrossRef] [PubMed]




| Isolated Macrophages and/or Macrophage Cell Lines | Inducers/Samples | Gene Expressed | Cytokines/Chemokines | Surface Markers | References |
|---|---|---|---|---|---|
| RAW264.7 | LPS + IFNγ | IL-1β, IL-6, TNF-α, iNOS, CCL2 (M1) Arg1, Ym1, IL-4, Mrc-1, Mgl1 (M2) | IL-1β, IL-6, TNF-α (M1) - | - | [15] |
| RAW264.7 | LPS (200 ng/mL) | - | TNF-α, IL-6, GM-CSF, IL-1α, G-CSF (M1), COX-2 (M1) - | CD86 (M1) CD206 (M2) | [16] |
| RAW264.7 | LPS (100 ng/mL) + IFNγ (2.5 ng/mL) | IL-1, IL-6, TNF-α (M1) - | - | CD86 (M1) | [17] |
| CD14+ isolated human peripheral blood monocytes | M-CSF/IFNγ (100 ng/mL) + M-CSF LPS (100 ng/mL) | IL-6 (M1) IL-10 (M2) | TNFα, IL-6 (M1) IL-10 (M2) | - | [18] |
| Peritoneal macrophage | LPS (20 ng/mL) + IFNγ (1 µg/mL)/IL-4 (20 ng/mL) | IL-1β, IL-6, TNFα, IL-12, iNOS (M1) IL-10, Fizz-1 (M2) | - IL-10, Fizz-1, Ym-1 (M2) | - | [19] |
| RAW264.7 | IL-4/IL13 (10 ng/mL) + Fenretinide | Fizz-1, PPARγ (M2) | STAT6 (M2) | CD206 (M2) | [20] |
| Peritoneal macrophage | LPS (100 ng/mL) + IFNγ (10 ng/mL)/IL-4 (200 ng/mL) | Irf5, iNOS (M1) Fizz-1, Irf4 (M2) | - | - | [21] |
| RAW264.7 ANA-1 THP-1 Murine peritoneal cells | LPS (500 ng/ mL)/IL-4 (10 ng/mL) + curcumin | CCL3, IL-1β (M1) Arg-1, Ym1, CD206 (M2) | MHCII, CCR7, TNF-α, (M1) MGL1/2 (M2) | CD80 (M1)- | [17] |
| RAW264.7 Bone marrow derived macrophage | LPS (100 ng/mL) + IL-4 (10 ng/mL)/10 ng/mL + Pentamethoxyflavanone | IL-1β, IL-6, TNF-α, iNOS (M1) Fizz-1, Ym-1, CD206 (M2) | - | CD11c (M1) CD206 (M2) | [22] |
| Murine peritoneal Macrophages RAW264.7 | LPS/IL-4 + Apegenin | CCR2, TNF-α, CCL3, CCL4, IL-1β, NOS2 (M1) Fizz-1, Ym-1, Arg-1, MGL1/2, CD163, CD206, IL-1r (M2) | TNF-α, CCL2, IL-1β, IL-6, IL-12, CCR7, MHCII (M1) IL-10, Arg-1, MGL1/2 (M2) | CD80 (M1) CD206 (M2) | [23] |
| RAW264.7 | Curcumin (25 µmol/mL) | IL-4, IL-13, MMR, Arg-1 (M2) | IL-4,IL-13, MMR and Arg-1 (M2) | - | [24] |
| Murine peritoneal macrophages | LPS (100 ng/mL) | - | iNOS, COX-2 (M1) | - | [25] |
| RAW264.7 | Tim-3 overexpressing | TNFα, IL-12, IFNγ, IL-17 (M1) IL-10 (M2) | - | - | [26] |
| Mouse peritoneal macrophages | LPS (10-100 ng/mL) + ginsenoside Rg3 | COX-2, iNOS, IL-1β, TNF-α (M1) Arg-1, IL-10, TGF-β, TNFα, Ym1 (M2) | - | - | [27] |
| RAW264.7 | LPS/Adipocytes-conditioned medium + Theaflavin-3, 3′-digallate | iNOS, TNF-α, IL-6, IL-1β, COX-2 (M1) Arg-1, PPARγ (M2) | TNF-α, IL-1β, IL-6, MCP1, CCR7 (M1) IL-10, STAT3 (M2) | CD11c, CD86 (M1) CD206, CD163 (M2) | [28] |
| Human and murine blood monocyte | Exosomal αvβ6 | - | CCL2/CCR2, STAT3 (M2) | CD204, CD163 (M2) | [29] |
| Microglia cell line N9 | LPS (0.1 µg/mL)/IL-4 (20 ng/mL) + Anthocyanins | IL-6, iNOS, IL-1β (M1) Ym-1, CCR-2, Arg-1, CX3CR1 (M2) | - | - | [30] |
| Primary microglia cells | IL-4 (10 ng/mL) + sevoflurane | Arg-1, Ym-1, IL-10 (M2) | - | - | [31] |
| Primary Kuffer cells Bone Marrow-derived macrophage (BMDM) | IL-4 | TNF-α, NOS2 (M1) Arg-1, IL-10, Retnlα (M2) | - | CD80 (M1) CD206, CD163 (M2) | [32] |
| Bone Marrow-derived macrophage (BMDM) treated mice (liver tissue) | LPS + IFNγ/IL-4 | iNOS, IL-12, TNFα (M1) Arg-1, Mrc-1, Ym-1 (M2) | CCL2, CCL3 (M1) Lyc6 (New classification) | - | [33] |
| CD14+ peripheral blood mononuclear cells | IFNγ (100 ng/mL)/IL-4 (10 ng/mL) + M-CSF (5 ng/mL) | - | TNF-α, IL-1β IL-1Rα, CCL18 (M1) | - | [34] |
| Primary microglia cells | Erythrocyte lysates + Sinomenine | iNOS, IL-1β, TNF-α (M1) IL-10, Arg-1 (M2) | - | - | [35] |
| BV2 microglia cells Primary microglia cell culture | LPS (200 ng/mL) + IFNγ (20 ng/mL)/IL-4 (10 ng/mL)/SSRI | IL-1β, IL-6, iNOS, TNFα (M1) IL-10 (M2) | IL-1β, TNF-α (M1) IL-10 (M2) | CD86 (M1) CD206 (M2) | [36] |
| RAW264.7 Bone Marrow-derived macrophage (BMDM) | LPS (10 ng/mL) + IFNγ (10 ng/mL)/ IL-4 (10 ng/mL) + isomeranzin | IL-1β, IL-6, TNF-α (M1 Raw 264.7) IL-1β, IL-6, iNOS, TNF-α (M1 BMDM) Arg-1, Fizz-1, Ym-1 (M2) | IL-1β, IL-6, TNF-α (M1) - | CD11, iNOS (M1) - | [37] |
| Primary microglia cells BV-2 cells | LPS (0.1-1 µg/mL) + Diogenin glucoside | iNOS, TNF-α, IL-6, IL-23, IL-17, IL-1β (M1) IL-10, IL-1Rα, G-CSF, SOC3, COX-1, COX-2, Arg-1, Fizz-1, Ym-1, Chi3l3 (M2) | TNF-α, IL-6 (M1) IL-10, IL-1Rα (M2) | CD206 (M2) | [38] |
| RAW264.7 | LPS (500 ng/mL) + IFNγ (20 ng/mL) | - | - | CD86 (M1) CD206 (M2) | [39] |
| Bone Marrow-derived macrophage (BMDM) | - | - | Arg-1 (M2) | CD206 (M2) | [40] |
| Peripheral blood CD14+ monocyte | GM-CSF (50 ng/mL)+LPS (1 ng/mL) + IFNγ (20 ng/mL)/M-CSF (100 ng/mL) + IL-4 (20 ng/mL) + pentacyclic tirterpene Lupeol | IL-1β, IL-12, TNF-α (M1) IL-10 (M2) | - | CD86 (M1)CD206 (M2) | [41] |
| Mouse peritoneal macrophages | LPS (1 µg/mL) + baicalin | TNFα,IL-23 (M1) IL-10, Arg-1 (M2) | - Fizz-1 (M2) | - | [42] |
| Inducers/Samples | Gene Expressed | Cytokines | Surface Markers | References | |
|---|---|---|---|---|---|
| Ipsilateral and contralateral hippocampi | CCl | IL-1β, IL-6, TNF-α, IFN-γ (M1) IL-10, IL-13, Arg-1, Fizz-1, Mrc-1, Ym-1 (M2) | IL-1β, IL-6, TNF-α, IFN-γ (M1) IL-4, IL-10, IL-13 (M2) | - | [43] |
| Brain tissue | Cuprizone + Progesterone | iNOS, MHC II, TNF-α (M1) Trem-2, Arg-1, TGF-β (M2) | IL-18 (M1)- | CD86 (M1) CD206 (M2) | [44] |
| Colonic tissue Peritoneal macrophage | DSS + LZ205 Alum + LZ205 | IL-1β, IL-6, TNF-α, CCL2, iNOS (M1) | IL-1β, IL-6, TNF-α, MPO, iNOS (M1) - | - | [15] |
| Tumor region at colonic tissue | APCmin/+ mice given high fat diet + Fenretinide | - | - | CD206 (M2) | [20] |
| Arteriosclerotic plaque | Irgm1−/− and ApoE−/− given western diet | - | iNOS (M1) Arg-1, Fizz-1 (M2) | CD16/32 (M1)- | [21] |
| Adipose tissue | High fat diet + Chrysin | - | IL-1β, TNF-α (M1) IL-10 (M2) | NOS2 (M1)- | [45] |
| Serum, BALF and Lung tissue | LPS/ Puncture (CLP) surgery + Pentamethoxyflavanone | IL-1β, IL-6, TNF-α (M1)- | IL-1β, IL-6, TNF-α (M1)- | CD11c (M1)- | [22] |
| Adipose tissue | High fat diet + Apigenin | CCL3, CCL4 (M1) Ym-1, Arg-1 (M2) | TNF-α, CCL2, IL-1β, IL-6, IL-12, CCR7, MHCII (M1) IL-10, MGL1/2, Arg-1 (M2) | CD80 (M1) CD206 (M2) | [23] |
| Myocardial tissue in heart injury | EAM (treatment) + Curcumin | IL-1β, iNOS (M1) MMR, Arg-1 (M2) | - | - | [24] |
| Liver tissue | TAA | - | IFN-γ, TNF-α, MCP-1, Iba-1, MHC II (M1) IL-4, TGF-β, Gal-3, Hsp25 (M2) | CD68 (M1) CD163, CD204 (M2) | [46] |
| Colonic tissue | TNBS + 4-methoxychocarpin | TNF, IL-1β, IL-17A, Arg-2 (M1) IL-10 (M2) | - | CD86 (M1)- | [25] |
| Intestinal macrophages isolated from intestinal lamina propria cells | DSS + Tim-3 (protein) | TNF, IL-1β, NOS2, IL-6, IL-12 (M1) Arg-1 (M2) | - | CD16/32 (M1) Dectin-1 (M2) | [26] |
| Benign and malignant tumor (breast cancer) | Canine mammary tumors | - | NOS2 (M1) | -CD206 (M2) | [47] |
| Blood monocyte Red pulp macrophage and monocyte in murine spleen Synovial tissue Joint lavage | Hemathrosis induction | - | MHC1, MHC2, Ly-6C (M1) | CD86 (M1) CD163 (M2) | [48] |
| Microglia cells isolated from myeloid cells | Alcohol | - | MHC II, iNOS (M1) MMR (M2) | CD86 CD16/32 (M1) CD206 (M2) | [49] |
| Perihematomal region of cerebral tissues | Blood injection into striatum | - | iNOS, IL-1β, TNF-α (M1) | - | [35] |
| Placenta cells Splenocytes | Maternal low protein diet | IL-12, IL-6, IL-10, IL-1β, NOS2 (M1) Mrc1(CD206), Mgl1(CD301) (M1) | TNF-α (M1) IL-6, TNF-α (M2b), CCL4 (M2) | CD274 (M1) CD163 (M2) | [50] |
| Liver tissue/serum/BALF Colonic tissue | LPS/DSS/TNBS + isomeranzin | IL-1β, IL-6, TNF-α, iNOS (M1) | IL-1β, IL-6, TNF-α (M1) | - | [37] |
| Colonic tissue | DSS + C.EDA | - | IL-1β, IL-6, TNF-α, IL-10 (M1) | CD11c (M1) CD206 (M2) | [39] |
| Colonic tissue | DSS + pentacyclic triterpene Lupeol | IL-12, IL-6, IL-1β, TNF-α, iNOS (M1)- | CD86 (M1) CD206 (M2) | [41] | |
| Mouse colonic lamina propria mononuclear cells | DSS + baicalin | TNF-α,IL-23 (M1) TNF-α, IL-23, IL-10, Arg-1 (M2) | - | - | [42] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, Y.-C.; Yang, G.; Lai, C.-S.; Weerawatanakorn, M.; Pan, M.-H. Chemopreventive Effects of Phytochemicals and Medicines on M1/M2 Polarized Macrophage Role in Inflammation-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2208. https://doi.org/10.3390/ijms19082208
Koh Y-C, Yang G, Lai C-S, Weerawatanakorn M, Pan M-H. Chemopreventive Effects of Phytochemicals and Medicines on M1/M2 Polarized Macrophage Role in Inflammation-Related Diseases. International Journal of Molecular Sciences. 2018; 19(8):2208. https://doi.org/10.3390/ijms19082208
Chicago/Turabian StyleKoh, Yen-Chun, Guliang Yang, Ching-Shu Lai, Monthana Weerawatanakorn, and Min-Hsiung Pan. 2018. "Chemopreventive Effects of Phytochemicals and Medicines on M1/M2 Polarized Macrophage Role in Inflammation-Related Diseases" International Journal of Molecular Sciences 19, no. 8: 2208. https://doi.org/10.3390/ijms19082208
APA StyleKoh, Y.-C., Yang, G., Lai, C.-S., Weerawatanakorn, M., & Pan, M.-H. (2018). Chemopreventive Effects of Phytochemicals and Medicines on M1/M2 Polarized Macrophage Role in Inflammation-Related Diseases. International Journal of Molecular Sciences, 19(8), 2208. https://doi.org/10.3390/ijms19082208