Miniaturized Biomedical Sensors for Enumeration of Extracellular Vesicles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure of GaN HEMT Biosensor
2.2. Surface Functionalization and Characterization
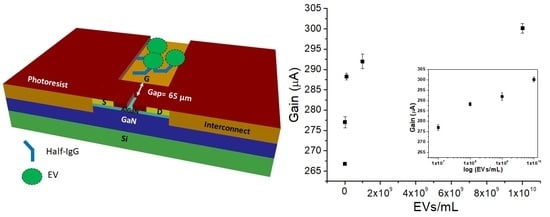
2.3. Detection and Enumeration of EVs
2.4. Sensor Regeneration
3. Materials and Methods
3.1. AlGaN/GaN HEMT Fabrication
3.2. Sensor Surface Functionalization
3.3. Cell Culture and EV Production and Characterization
3.4. Sensor Regeneration
3.5. Sensor Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Ann. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Marine, M.; Liliana, P.; Maëva, V.; Ramaroson, A.; Gilles, S.; Carmen, M.M. Extracellular Vesicles: Mechanisms in Human Health and Disease. Antioxid. Redox Signal. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.G.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Antovic, J.; Egberg, N.; Hansson, M.; Jörneskog, G.; Hultenby, K.; Wallén, H. A multicolor flow cytometric assay for measurement of platelet-derived microparticles. Thromb. Res. 2010, 125, e110–e116. [Google Scholar] [CrossRef] [PubMed]
- Garza-Licudine, E.; Deo, D.; Yu, S.; Uz-Zaman, A.; Dunbar, W.B. Portable nanoparticle quantization using a resizable nanopore instrument—The IZON qNanoTM. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar]
- Chen, C.; Lin, B.-R.; Hsu, M.-Y.; Cheng, C.-M. Paper-based Devices for Isolation and Characterization of Extracellular Vesicles. J. Vis. Exp. 2015, 98, 52722. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-I.; Li, B.-R.; Chen, Y.-T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Arya, S.K.; Wong, C.C.; Jeon, Y.J.; Bansal, T.; Park, M.K. Advances in Complementary-Metal–Oxide–Semiconductor-Based Integrated Biosensor Arrays. Chem. Rev. 2015, 115, 5116–5158. [Google Scholar] [CrossRef] [PubMed]
- Elnathan, R.; Kwiat, M.; Pevzner, A.; Engel, Y.; Burstein, L.; Khatchtourints, A.; Lichtenstein, A.; Kantaev, R.; Patolsky, F. Biorecognition Layer Engineering: Overcoming Screening Limitations of Nanowire-Based FET Devices. Nano Lett. 2012, 12, 5245–5254. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye Screening Length on Nanowire Field Effect Transistor Sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, E.; Vacic, A.; Rajan, N.K.; Criscione, J.M.; Park, J.; Ilic, B.R.; Mooney, D.J.; Reed, M.A.; Fahmy, T.M. Label-free biomarker detection from whole blood. Nat. Nano 2010, 5, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Sarangadharan, I.; Regmi, A.; Chen, Y.-W.; Hsu, C.-P.; Chang, W.-H.; Lee, G.-Y.; Chyi, J.-I.; Chen, C.-C.; Shiesh, S.-C.; et al. Beyond the Debye length in high ionic strength solution: Direct protein detection with field-effect transistors (FETs) in human serum. Sci. Rep. 2017, 7, 5256. [Google Scholar] [CrossRef] [PubMed]
- Sarangadharan, I.; Regmi, A.; Chen, Y.-W.; Hsu, C.-P.; Chen, P.-C.; Chang, W.-H.; Lee, G.-Y.; Chyi, J.-I.; Shiesh, S.-C.; Lee, G.-B.; et al. High sensitivity cardiac troponin I detection in physiological environment using AlGaN/GaN High Electron Mobility Transistor (HEMT) Biosensors. Biosens. Bioelectr. 2018, 100, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Chen, Y.-W.; Sarangadharan, I.; Hsu, C.-P.; Chen, C.-C.; Shiesh, S.-C.; Lee, G.-B.; Wang, Y.-L. Editors’ Choice—Field-Effect Transistor-Based Biosensors and a Portable Device for Personal Healthcare. ECS J. Solid State Sci. Technol. 2017, 6, Q71–Q76. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Reátegui, E.; van der Vos, K.E.; Lai, C.P.; Zeinali, M.; Atai, N.A.; Aldikacti, B.; Floyd, F.P.; Khankhel, H.A.; Thapar, V.; Hochberg, F.H.; et al. Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles. Nat. Commun. 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revenfeld, A.L.S.; Bæk, R.; Nielsen, M.H.; Stensballe, A.; Varming, K.; Jørgensen, M. Diagnostic and Prognostic Potential of Extracellular Vesicles in Peripheral Blood. Clin. Ther. 2014, 36, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Nickenig, G.; Werner, N. Extracellular Vesicles in Cardiovascular Disease. Circ. Res. 2017, 120, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Szajnik, M.; Derbis, M.; Lach, M.; Patalas, P.; Michalak, M.; Drzewiecka, H.; Szpurek, D.; Nowakowski, A.; Spaczynski, M.; Baranowski, W.; et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol. Obstet. 2013. [Google Scholar] [CrossRef]
- Baran, J.; Baj-Krzyworzeka, M.; Weglarczyk, K.; Szatanek, R.; Zembala, M.; Barbasz, J.; Czupryna, A.; Szczepanik, A.; Zembala, M. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Can. Immunol. Immunother. 2010, 59, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, K.S.; Park, Y.S.; Kang, Y.H.; Lee, Y.J.; Lee, K.R.; Kim, H.K.; Ryu, K.W.; Bae, J.M.; Kim, S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: Possible role of a metastasis predictor. Eur. J. Can. 2003, 39, 184–191. [Google Scholar] [CrossRef]
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal MicroRNA: A Diagnostic Marker for Lung Cancer. Clin. Lung Can. 2009, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Sarangadharan, I.; Sukesan, R.; Hseih, C.-Y.; Lee, G.-Y.; Chyi, J.-I.; Wang, Y.-L. High-field modulated ion-selective field-effect-transistor (FET) sensors with sensitivity higher than the ideal Nernst sensitivity. Sci. Rep. 2018, 8, 8300. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Lin, H.-Y.; Liao, C.-Z.; Chyi, J.-I. Growth and Characterization of Crack-Free Semi-Polar (1-101) GaN on 7°-off (001) Si Substrates by Metal-Organic Chemical Vapor Deposition. ECS J. Solid State Sci. Technol. 2013, 2, N3001–N3005. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Lin, C.-W.; Kao, H.-L.; Lee, G.-Y.; Chyi, J.-I.; Chuang, H.-W.; Chang, K.-J.; Gau, Y.-T. A gold-free fully copper metalized AlGaN/GaN power HEMTs on Si substrate. Microelectron. Reliab. 2012, 52, 2556–2560. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Chen, P.-C.; Pulikkathodi, A.K.; Hsiao, Y.-H.; Chen, C.-C.; Wang, Y.-L. A Package Technology for Miniaturized Field-Effect Transistor-Based Biosensors and the Sensor Array. ECS J. Solid State Sci. Technol. 2017, 6, Q63–Q67. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulikkathodi, A.K.; Sarangadharan, I.; Lo, C.-Y.; Chen, P.-H.; Chen, C.-C.; Wang, Y.-L. Miniaturized Biomedical Sensors for Enumeration of Extracellular Vesicles. Int. J. Mol. Sci. 2018, 19, 2213. https://doi.org/10.3390/ijms19082213
Pulikkathodi AK, Sarangadharan I, Lo C-Y, Chen P-H, Chen C-C, Wang Y-L. Miniaturized Biomedical Sensors for Enumeration of Extracellular Vesicles. International Journal of Molecular Sciences. 2018; 19(8):2213. https://doi.org/10.3390/ijms19082213
Chicago/Turabian StylePulikkathodi, Anil Kumar, Indu Sarangadharan, Chiao-Yun Lo, Po-Hsuan Chen, Chih-Chen Chen, and Yu-Lin Wang. 2018. "Miniaturized Biomedical Sensors for Enumeration of Extracellular Vesicles" International Journal of Molecular Sciences 19, no. 8: 2213. https://doi.org/10.3390/ijms19082213