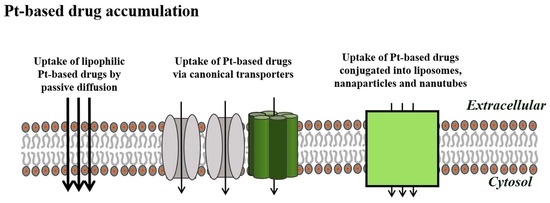
Facilitating the Cellular Accumulation of Pt-Based Chemotherapeutic Drugs
Abstract
:1. Introduction
2. Apoptotic Cell Death and Drug Resistance
3. Cellular Cisplatin Accumulation
3.1. Copper Transporters and ATPases
3.2. The Volume-Regulated Anion Channel (VRAC) Family
4. Alternative Pathways for Accumulation of Cisplatin
4.1. B12 Conjugates
4.2. Polyethylene (PEG) and Human Albumin (HSA) Conjugates
4.3. Liposome Conjugates
4.4. Folate Derivatives
4.5. Integrins
4.6. Passive Diffusion
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, B.V.L.; Krigas, S.T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Lambert, I.H. Ion channels and transporters in the development of drug resistance in cancer cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- El, M.G.; Le, T.C.; Batty, G.N.; Faivre, S.; Raymond, E. Markers involved in resistance to cytotoxics and targeted therapeutics in pancreatic cancer. Cancer Treat. Rev. 2009, 35, 167–174. [Google Scholar]
- Tamburrino, A.; Piro, G.; Carbone, C.; Tortora, G.; Melisi, D. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front. Pharmacol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, M.; Delgado, Y.; Sharma, R.K.; Sharma, S.; Guzman, S.L.P.L.; Tinoco, A.D.; Griebenow, K. Inducing cell death in vitro in cancer cells by targeted delivery of cytochrome c via a transferrin conjugate. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.B.; Safaei, R.; Larson, C.A.; Sailor, M.J. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol. 2010, 77, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Sprowl, J.A.; Ness, R.A.; Sparreboom, A. Polymorphic transporters and platinum pharmacodynamics. Drug Metab. Pharmacokinet. 2013, 28, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Larson, C.A.; Safaei, R.; Howell, S.B. Molecular modulation of the copper and cisplatin transport function of CTR1 and its interaction with IRS-4. Biochem. Pharmacol. 2014, 90, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Jandial, D.D.; Farshchi-Heydari, S.; Larson, C.A.; Elliott, G.I.; Wrasidlo, W.J.; Howell, S.B. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin. Cancer Res. 2009, 15, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.G.; Larson, C.A.; Adams, P.L.; Abada, P.B.; Pesce, C.E.; Safaei, R.; Howell, S.B. Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol. Pharmacol. 2011, 79, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Bompiani, K.M.; Tsai, C.Y.; Achatz, F.P.; Liebig, J.K.; Howell, S.B. Copper transporters and chaperones CTR1, CTR2, ATOX1, and CCS as determinants of cisplatin sensitivity. Metallomics 2016, 8, 951–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadini-Buoninsegni, F.; Bartolommei, G.; Moncelli, M.R.; Inesi, G.; Galliani, A.; Sinisi, M.; Losacco, M.; Natile, G.; Arnesano, F. Translocation of platinum anticancer drugs by human copper ATPases ATP7A and ATP7B. Angew. Chem. Int. Ed. Engl. 2014, 53, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Kalayda, G.V.; Wagner, C.H.; Buss, I.; Reedijk, J.; Jaehde, U. Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Harrach, S.; Ciarimboli, G. Role of transporters in the distribution of platinum-based drugs. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaei, R.; Adams, P.L.; Maktabi, M.H.; Mathews, R.A.; Howell, S.B. The CXXC motifs in the metal binding domains are required for ATP7B to mediate resistance to cisplatin. J. Inorg. Biochem. 2012, 110, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaei, R.; Adams, P.L.; Mathews, R.A.; Manorek, G.; Howell, S.B. The role of metal binding and phosphorylation domains in the regulation of cisplatin-induced trafficking of ATP7B. Metallomics 2013, 5, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Nozaki, S.; Kitahara, H.; Ohara, T.; Kato, K.; Kawashiri, S.; Yamamoto, E. Copper efflux transporter (ATP7B) contributes to the acquisition of cisplatin-resistance in human oral squamous cell lines. Oncol. Rep. 2007, 18, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, P.V.; Klomp, L.W. Posttranslational regulation of copper transporters. J. Biol. Inorg. Chem. 2010, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, W.; Zhao, X.; Wang, P. MiR-133a enhances the sensitivity of Hep-2 cells and vincristine-resistant Hep-2v cells to cisplatin by downregulating ATP7B expression. Int. J. Mol. Med. 2016, 37, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Chen, Z.; Li, R. MicroRNA-133a inhibits proliferation and invasion, and induces apoptosis in gastric carcinoma cells via targeting fascin actin-bundling protein 1. Mol. Med. Rep. 2015, 12, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shanbhag, V.; Wang, Y.; Lee, J.; Petris, M. A role for The ATP7A copper transporter in tumorigenesis and cisplatin resistance. J. Cancer 2017, 8, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.D.; Wang, N.D.; Li, X.L.; Yan, J.; Tang, J.H.; Zhao, X.H.; Zhang, Z. Toosendanin mediates cisplatin sensitization through targeting Annexin A4/ATP7A in non-small cell lung cancer cells. J. Nat. Med. 2018, 72, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.H.; Dam, C.S.; Sturup, S.; Lambert, I.H. Dual role of LRRC8A-containing transporters on cisplatin resistance in human ovarian cancer cells. J. Inorg. Biochem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Benedetti, V.; Cossa, G.; Assaraf, Y.G.; Bram, E.; Gatti, L.; Corna, E.; Carenini, N.; Colangelo, D.; Howell, S.B.; et al. Increased levels and defective glycosylation of MRPs in ovarian carcinoma cells resistant to oxaliplatin. Biochem. Pharmacol. 2010, 79, 1108–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, E.K.; Sorensen, B.H.; Sauter, D.P.; Lambert, I.H. Role of volume-regulated and calcium-activated anion channels in cell volume homeostasis, cancer and drug resistance. Channels 2015, 9, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Lang, F. Mechanisms and significance of cell volume regulation. J. Am. Coll. Nutr. 2007, 26, 613S–623S. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine—From organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Lutter, D.; Ullrich, F.; Lueck, J.C.; Kempa, S.; Jentsch, T.J. Selective transport of neurotransmitters and modulators by distinct volume-regulated LRRC8 anion channels. J. Cell Sci. 2017, 130, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Mongin, A.A. Volume-regulated anion channel—A frenemy within the brain. Pflugers Arch. 2016, 468, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Qiu, Z.; Dubin, A.E.; Murthy, S.E.; Florendo, M.N.; Mason, D.E.; Mathur, J.; Cahalan, S.M.; Peters, E.C.; Montal, M.; et al. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell 2016, 164, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Voss, F.K.; Ullrich, F.; Munch, J.; Lazarow, K.; Lutter, D.; Mah, N.; Andrade-Navarro, M.A.; von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.H.; Thorsteinsdottir, A.A.; Lambert, I.H. Acquired cisplatin resistance in human ovarian cancer A2780 cells correlates with shift in taurine homeostasis and ability to volume regulate. Am. J. Physiol. 2014, 307, C1071–C1080. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.H.; Nielsen, D.N.; Torsteinsdottir, U.A.; Hoffmann, E.K.; Lambert, I.H. Down-regulation of LRRC8A protects human ovarian and alveolar carcinima cells against cisplatin-induced expression of p53, MDM2, p21WAF1/Cip1 and Caspase-9/-3 activation. Am. J. Physiol. Cell Physiol. 2016, 310, C857–C873. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Lutter, D.; Guyader, C.; Gerhards, N.M.; Ullrich, F.; Elger, D.A.; Kucukosmanoglu, A.; Xu, G.; Voss, F.K.; Reincke, S.M.; et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015, 34, 2993–3008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kefauver, J.M.; Saotome, K.; Dubin, A.E.; Pallesen, J.; Cottrell, C.A.; Cahalan, S.M.; Qiu, Z.; Homh, G.; Crowley, C.S.; Whitwam, T.; et al. Structure of the human volume regulated anion channel. BioRxiv 2018. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. Bioessays 2012, 34, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin channels are not gap junction hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyzinski-Garcia, M.C.; Rudkouskaya, A.; Mongin, A.A. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J. Physiol. 2014, 592, 4855–4862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentsch, T.J.; Lutter, D.; Planells-Cases, R.; Ullrich, F.; Voss, F.K. VRAC: Molecular identification as LRRC8 heteromers with differential functions. Pflugers Arch. 2016, 468, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.L.; Wilson, C.S.; Mongin, A.A. Molecular composition and heterogeneity of the LRRC8-containing swelling-activated osmolyte channels in primaryastrocytes. J. Physiol. 2017, 595, 6939–6951. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.A.; Andersen, E.C.; Hansen, C.F.; Klausen, T.K.; Hougaard, C.; Lambert, I.H.; Hoffmann, E.K. Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: Role of chloride channels. Am. J. Physiol. Cell Physiol. 2010, 298, C14–C25. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.L.; Shimizu, T.; Ise, T.; Numata, T.; Kohno, K.; Okada, Y. Impaired activity of volume-sensitive Cl- channel is involved in cisplatin resistance of cancer cells. J. Cell Physiol. 2007, 211, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Min, X.J.; Li, H.; Hou, S.C.; He, W.; Liu, J.; Hu, B.; Wang, J. Dysfunction of volume-sensitive chloride channels contributes to cisplatin resistance in human lung adenocarcinoma cells. Exp. Biol. Med. 2011, 236, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, S.; McDonald, T.; Gardner, P.; Kang, N.; Gupta, S. Abnormal chloride conductance in multidrug resistant HL60/AR cells. Cancer Lett. 1992, 66, 83–89. [Google Scholar] [CrossRef]
- Kang, X.; Xiao, H.H.; Song, H.Q.; Jing, X.B.; Yan, L.S.; Qi, R.G. Advances in drug delivery system for platinum agents based combination therapy. Cancer Biol. Med. 2015, 12, 362–374. [Google Scholar] [PubMed]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug Delivery Strategies for Platinum-Based Chemotherapy. ACS Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, C.; Wang, A.Z.; Lin, W. Application of liposomal technologies for delivery of platinum analogs in oncology. Int. J. Nanomed. 2013, 8, 3309–3319. [Google Scholar]
- Tran, M.T.Q.; Stürup, S.; Lambert, I.H.; Gammelgaard, B.; Furger, E.; Waibel, R.; Alberto, R. Cellular uptake of metallated cobalamins. Metallomics 2016, 8, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tastesen, H.S.; Holm, J.B.; Møller, J.; Poulsen, K.A.; Møller, C.; Stürup, S.; Hoffmann, E.K.; Lambert, I.H. Pinpointing differences in cisplatin-induced apoptosis in adherent and non-adherent cancer cells. Cell Physiol. Biochem. 2010, 26, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N. Physiological and molecular aspects of cobalamin transport. Subcell. Biochem. 2012, 56, 347–367. [Google Scholar] [PubMed]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.; Nexo, E.; Moestrup, S.K. Vitamin B12 transport from food to the body’s cells—A sophisticated, multistep pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Fazen, C.H.; Valentin, D.; Fairchild, T.J.; Doyle, R.P. Oral delivery of the appetite suppressing peptide hPYY (3-36) through the vitamin B12 uptake pathway. J. Med. Chem. 2011, 54, 8707–8711. [Google Scholar] [CrossRef] [PubMed]
- Petrus, A.K.; Vortherms, A.R.; Fairchild, T.J.; Doyle, R.P. Vitamin B12 as a carrier for the oral delivery of insulin. ChemMedChem 2007, 2, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Petrus, A.K.; Allis, D.G.; Smith, R.P.; Fairchild, T.J.; Doyle, R.P. Exploring the implications of vitamin B12 conjugation to insulin on insulin receptor binding. ChemMedChem 2009, 4, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Petrus, A.K.; Fairchild, T.J.; Doyle, R.P. Traveling the vitamin B12 pathway: Oral delivery of protein and peptide drugs. Angew. Chem. Int. Ed. Engl. 2009, 48, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Lildballe, D.L.; Mutti, E.; Birn, H.; Nexo, E. Maximal load of the vitamin B12 transport system: A study on mice treated for four weeks with high-dose vitamin B12 or cobinamide. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Garmann, D.; Warnecke, A.; Kalayda, G.V.; Kratz, F.; Jaehde, U. Cellular accumulation and cytotoxicity of macromolecular platinum complexes in cisplatin-resistant tumor cells. J. Control. Release 2008, 131, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hagan, C.T.; Min, Y.; Foley, H.; Tian, X.; Yang, F.; Mi, Y.; Au, K.M.; Medik, Y.; Roche, K.; et al. Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials 2018, 169, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, N.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical activity of the liposomal cisplatin lipoplatin in ovarian cancer. Clin. Cancer Res. 2014, 20, 5496–5506. [Google Scholar] [CrossRef] [PubMed]
- Farhat, F.S.; Temraz, S.; Kattan, J.; Ibrahim, K.; Bitar, N.; Haddad, N.; Jalloul, R.; Hatoum, H.A.; Nsouli, G.; Shamseddine, A.I. A phase II study of lipoplatin (liposomal cisplatin)/vinorelbine combination in HER-2/neu-negative metastatic breast cancer. Clin. Breast. Cancer 2011, 11, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, G.P.; Boulikas, T. Lipoplatin formulation, review article. J. Drug Deliv. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Garrido, M.J. Liposomes, a promising strategy for clinical application of platinum derivatives. Expert Opin. Drug Deliv. 2013, 10, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, K.S.; Lippard, S.J. Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting. Dalton Trans. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronov, O.; Horowitz, A.T.; Gabizon, A.; Gibson, D. Folate-targeted PEG as a potential carrier for carboplatin analogs. Synthesis and in vitro studies. Bioconjug Chem. 2003, 14, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Liu, Z.; Thomale, J.; Dai, H.; Lippard, S.J. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 2008, 130, 11467–11476. [Google Scholar] [CrossRef] [PubMed]
- Alavizadeh, S.H.; Akhtari, J.; Badiee, A.; Golmohammadzadeh, S.; Jaafari, M.R. Improved therapeutic activity of HER2 Affibody-targeted cisplatin liposomes in HER2-expressing breast tumor models. Expert Opin. Drug Deliv. 2016, 13, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Lv, P.; Zhang, P. Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol. Lett. 2015, 9, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, C.Z.; Fan, H.J.; Zhang, C.J.; Zhang, H.W.; Lv, M.H.; Cui, S.D. Adual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncol. Lett. 2014, 8, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.H.; Rasmussen, L.J.H.; Broberg, B.S.; Klausen, T.K.; Sauter, D.P.R.; Lambert, I.H.; Asberg, A.; Hoffmann, E.K. Integrin B1, osmosensing, and chemoresistance in mouse ehrlich carcinoma cells. Cell. Physiol. Biochem. 2015, 36, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Koberle, B.; Tomicic, M.T.; Usanova, S.; Kaina, B. Cisplatin resistance: Preclinical findings and clinical implications. Biochim. Biophys. Acta 2010, 1806, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, B.H.; Werth, P.; Lambert, I.H.; Bednarski, P.J. In vitro evaluation of the enantiomeric R- and S-1, 1′-binaphthyl-2, 2′-diaminodichlorido-Pt(ii) complexes in human Burkitt lymphoma cells: Emphasis on cellular accumulation, cytotoxicity, DNA binding, and ability to induce apoptosis. Metallomics 2018, 10, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Bombard, S.; Gariboldi, M.B.; Monti, E.; Gabano, E.; Gaviglio, L.; Ravera, M.; Osella, D. Biological activity of enantiomeric complexes [PtCl(2)L (2)] (L (2) is aromatic bisphosphanes and aromatic diamines). J. Biol. Inorg. Chem. 2010, 15, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Gabano, E.; Gama, S.; Mendes, F.; Gariboldi, M.B.; Monti, E.; Bombard, S.; Bianco, S.; Ravera, M. Study of the synthesis, antiproliferative properties, and interaction with DNA and polynucleotides of cisplatin-like Pt(II) complexes containing carcinogenic polyaromatic amines. J. Biol. Inorg. Chem. 2013, 18, 791–801. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, I.H.; Sørensen, B.H. Facilitating the Cellular Accumulation of Pt-Based Chemotherapeutic Drugs. Int. J. Mol. Sci. 2018, 19, 2249. https://doi.org/10.3390/ijms19082249
Lambert IH, Sørensen BH. Facilitating the Cellular Accumulation of Pt-Based Chemotherapeutic Drugs. International Journal of Molecular Sciences. 2018; 19(8):2249. https://doi.org/10.3390/ijms19082249
Chicago/Turabian StyleLambert, Ian Henry, and Belinda Halling Sørensen. 2018. "Facilitating the Cellular Accumulation of Pt-Based Chemotherapeutic Drugs" International Journal of Molecular Sciences 19, no. 8: 2249. https://doi.org/10.3390/ijms19082249