Chitosan-Based Nanofibrous Membrane Unit with Gradient Compositional and Structural Features for Mimicking Calcified Layer in Osteochondral Matrix
Abstract
:1. Introduction
2. Results and Discussion
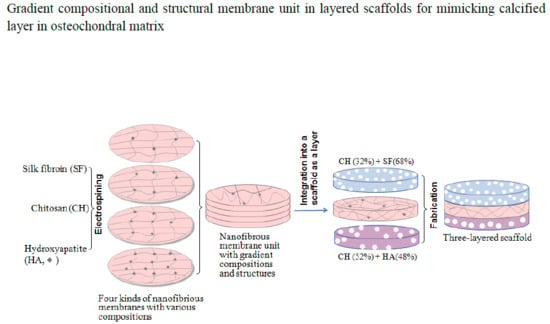
2.1. Nanofibrous Membranes Units
2.2. Fabrication of Layered Scaffolds
2.3. Permeable Properties of Mimetic Calcified Layer
2.4. Cell Culture
2.5. Matrix Deposition Assessment
3. Materials and Methods
3.1. Materials
3.2. Preparation of Silk Fibroin
3.3. Synthesis of Hydroxyapatite Nanoparticles
3.4. Preparation of Nanofibrous Membranes
3.5. Preparation of Layered Scaffolds
3.6. Characterization
3.7. Permeability Evaluation
3.8. Cell Culture
3.9. Matrix Deposition Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, J.; Zhu, L.; Morsi, Y.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Superelastic, superabsorbent and 3D nanofiber-assembled scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2016, 142, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Chen, J.; Pan, P.; Zhang, Y.; Hu, Y.; Liu, X.; Shi, X.; Zhang, Q. Bioinspired double polysaccharides-based nanohybrid scaffold for bone tissue engineering. Colloids Surf. B Biointerfaces 2016, 147, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Benetti, E.M.; Gunnewiek, M.K.; van Blitterswijk, C.A.; Vancso, G.J.; Moroni, L. Mimicking natural cell environments: Design, fabrication and application of bio-chemical gradients on polymeric biomaterial substrates. J. Mater. Chem. B. 2016, 4, 4244–4257. [Google Scholar] [CrossRef]
- Yan, L.P.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Current concepts and challenges in osteochondral tissue engineering and regenerative medicine. ACS Biomater. Sci. Eng. 2015, 1, 183–200. [Google Scholar] [CrossRef] [Green Version]
- Dormer, N.H.; Berkland, C.J.; Detamore, M.S. Emerging techniques in stratified designs and continuous gradients for tissue engineering of interfaces. Ann. Biomed. Eng. 2010, 38, 2121–2141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, A.; Ateshian, G.A.; Guo, X.E.; Hung, C.T.; Lu, H.H. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann. Biomed. Eng. 2010, 38, 2183–2196. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Mosher, C.Z.; Boushell, M.K.; Lu, H.H. Engineering complex orthopaedic tissues via strategic biomimicry. Ann. Biomed. Eng. 2015, 43, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ying, Z.; Duan, X.; Tan, H.; Yang, B.; Guo, L.; Chen, G.; Dai, G.; Ma, Z.; Yang, L. Histomorphometric analysis of adult articular calcified cartilage zone. J. Struct. Biol. 2009, 168, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Matsiko, A.; Dickson, G.R.; O’Brien, F.J.; Gleeson, J.P. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014, 10, 1996–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanarian, N.T.; Haney, N.M.; Burga, R.A.; Lu, H.H. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials 2012, 33, 5247–5258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da, H.; Jia, S.J.; Meng, G.L.; Cheng, J.H.; Zhou, W.; Xiong, Z.; Mu, Y.J.; Liu, J. The impact of compact layer in biphasic scaffold on osteochondral tissue engineering. PLoS ONE 2013, 8, e54838. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, M.; Xu, B.; Zhang, J.; Zhao, Y.; Ji, S.; Wang, L.; Wang, L.; Li, X.; Kong, D.; et al. Integrated trilayered silk fibroin scaffold for osteochondral differentiation of adipose-derived stem cells. ACS Appl. Mater. Interfaces 2014, 6, 16696–16705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, H.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S.; Wang, A. Fabrication and in vitro evaluation of an articular cartilage extracellular matrix-hydroxyapatite bilayered scaffold with low permeability for interface tissue engineering. Biomed. Eng. Online 2014, 13, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Ramesh, A.; Brady, R.T.; Brama, P.A.J.; Kearney, C.; Gleeson, J.P.; O’Brien, F.J. Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials 2016, 87, 69–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levingstone, T.J.; Thompson, E.; Matsiko, A.; Schepens, A.; Gleeson, J.P.; O’Brien, F.J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016, 32, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galperin, A.; Oldinski, R.A.; Florczyk, S.J.; Bryers, J.D.; Zhang, M.Q.; Ratner, B.D. Integrated bi-layered scaffold for osteochondral tissue engineering. Adv. Healthcare Mater. 2013, 2, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Seidi, A.; Ramalingam, M.; Elloumi-Hannachi, I.; Ostrovidov, S.; Khademhosseini, A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011, 7, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Liverani, L.; Roether, J.A.; Nooeaid, P.; Trombetta, M.; Schubert, D.W.; Boccaccini, A.R. Simple fabrication technique for multilayered stratified composite scaffolds suitable for interface tissue engineering. Mater. Sci. Eng. A 2012, 557, 54–58. [Google Scholar] [CrossRef]
- Yunos, D.M.; Ahmad, Z.; Salih, V.; Boccaccini, A.R. Stratified scaffolds for osteochondral tissue engineering applications: Electrospun PDLLA nanofibre coated Bioglass®-derived foams. J. Biomater. Appl. 2013, 27, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Sandri, M.; Landi, E.; Pressato, D.; Francioli, S.; Quarto, R.; Martin, I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 2008, 29, 3539–3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampieri, A.; Sprio, S.; Sandri, M.; Valentini, F. Mimicking natural bio-mineralization processes: A new tool for osteochondral scaffold development. Triends Biotechnol. 2011, 29, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Z.C.; Lin, C.J. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nano-composite for use in biomedical material. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Zhu, F.; Li, F.; Huang, Q. Synthesis and characterization of stimuli-responsive poly (2-dimethylamino-ethylmethacrylate)-grafted chitosan microcapsule for controlled pyraclostrobin release. Int. J. Mol. Sci. 2018, 19, 854. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.T.; Jung, Y.J.; Ryu, S.C.; Son, H.J.; Kim, Y.G. Preparation of a porous chitosan/fibroin-hydroxyapatite composite matrix for tissue engineering. Macromol. Res. 2007, 15, 65–73. [Google Scholar] [CrossRef]
- Chen, J.P.; Chen, S.H.; Lai, G.J. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res. Lett. 2012, 7, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Sultan, M.T.; Kim, S.H.; Kumar, V.; Yeon, Y.K.; Lee, O.J.; Park, C.H. Artificial auricular cartilage using silk fibroin and polyvinyl alcohol hydrogel. Int. J. Mol. Sci. 2017, 18, 1707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Hina, S.; Zhang, Y.; Wang, H. Characterization of polymeric solutions: A brief overview. Rev. Adv. Mater. Sci. 2014, 36, 165–176. [Google Scholar]
- Chen, C.H.; Kuo, C.Y.; Wang, Y.J.; Chen, J.P. Dual function of glucosamine in gelatin/hyaluronic acid cryogel to modulate scaffold mechanical properties and to maintain chondrogenic phenotype for cartilage tissue engineering. Int. J. Mol. Sci. 2016, 17, 1957. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.; Jiang, C.C. Repair of articular cartilage defects: Review and perspectives. J. Formos. Med. Assoc. 2009, 108, 87–101. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Christakiran, M.J.; Reardon, P.J.T.; Konwarh, R.; Knowles, J.C.; Mandal, B.B. Mimicking hierarchical complexity of the osteochondral interface using electrospun silk bioactive glass composites. ACS Appl. Mater. Interfaces 2017, 9, 8000–8013. [Google Scholar]
- Wan, Y.; Wen, D. Preparation and characterization of porous conducting poly (DL-lactide) composite membranes. J. Membr. Sci. 2005, 246, 193–201. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, J.J.; Liu, J.Y.; Luo, Y.; Wan, Y. Fabrication and characterization of layered chitosan/silk fibroin/nano-hydroxyapatite scaffolds with designed composition and mechanical properties. Biomed. Mater. 2015, 10, 045013. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Vipulanandan, C. Effect of pH, sulfate and sodium on the EDTA titration of calcium. Cem. Concr. Res. 2003, 33, 621–627. [Google Scholar] [CrossRef]
- Xie, Y.H.; Yu, H.J.; Ou, Y.N.; Li, C.D. Determination of phosphorus content in LiFePO4 by improved gravimetric method. Mater. Res. Innov. 2015, 19, 1285–1288. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, Y.; Zhang, J.; Yin, D.; Cheng, W. Manufacture of layered collagen/chitosan-polycaprolactone scaffoldswith biomimetic microarchitecture. Colloids Surf. B Biointerfaces 2014, 113, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wan, Y.; Cao, X.; Wu, Q. Proliferation of chondrocytes on porous poly (DL-lactide)/chitosan scaffolds. Acta Biomater. 2008, 4, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, J.; Wu, J.; Zhang, J.; Wan, Y.; Wu, H. Injectable hydrogels embedded with alginate microspheres for controlled delivery of bone morphogenetic protein-2. Biomed. Mater. 2016, 11, 025010. [Google Scholar] [CrossRef] [PubMed]








| Sample Name | Chitosan (wt%) | SF (wt%) | HA (wt%) | PEO (wt%) | Spinning Time (min) | Average Thickness (μm) | Average Diameter of Fibers (nm) | Average Pore Size (μm) (b) | Average Porosity (%) (c) |
|---|---|---|---|---|---|---|---|---|---|
| NFM(1) (a) | 36 | 50 | 10 | 4 | 265 | 157.6 ± 6.84 | 212.6 ± 23.54 | 2.8 ± 0.53 | 13.2 ± 1.64 |
| NFM(2) | 40 | 39 | 17 | 4 | 220 | 148.2 ± 8.13 | 231.2 ± 13.39 | 4.53 ± 0.92 | 18.4 ± 2.16 |
| NFM(3) | 44 | 28 | 24 | 4 | 175 | 153.1 ± 7.09 | 250.8 ± 11.71 | 6.62 ± 1.03 | 24.5 ± 2.71 |
| NFM(4) | 48 | 17 | 31 | 4 | 150 | 159.9 ± 9.61 | 267.4 ± 10.83 | 9.05 ± 1.14 | 31.7 ± 3.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Fang, Q.; Yu, X.; Wan, Y.; Xiao, B. Chitosan-Based Nanofibrous Membrane Unit with Gradient Compositional and Structural Features for Mimicking Calcified Layer in Osteochondral Matrix. Int. J. Mol. Sci. 2018, 19, 2330. https://doi.org/10.3390/ijms19082330
Liu J, Fang Q, Yu X, Wan Y, Xiao B. Chitosan-Based Nanofibrous Membrane Unit with Gradient Compositional and Structural Features for Mimicking Calcified Layer in Osteochondral Matrix. International Journal of Molecular Sciences. 2018; 19(8):2330. https://doi.org/10.3390/ijms19082330
Chicago/Turabian StyleLiu, Jiaoyan, Qing Fang, Xiaofeng Yu, Ying Wan, and Bo Xiao. 2018. "Chitosan-Based Nanofibrous Membrane Unit with Gradient Compositional and Structural Features for Mimicking Calcified Layer in Osteochondral Matrix" International Journal of Molecular Sciences 19, no. 8: 2330. https://doi.org/10.3390/ijms19082330