Myeloid-Derived Suppressor Cells as a Regulator of Immunity in Organ Transplantation
Abstract
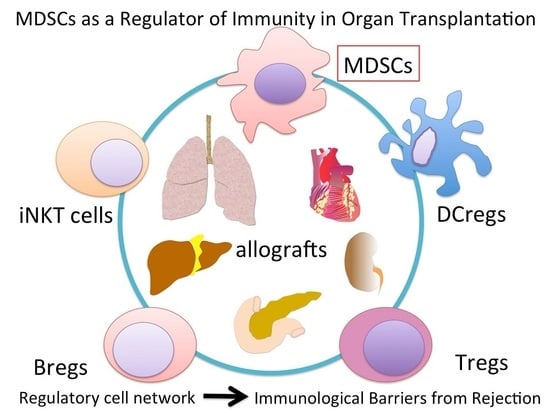
:1. Introduction
2. Definition and Characteristics of MDSCs
3. Effector of MDSCs
3.1. Inducible NO Synthase (iNOS)
3.2. Arginase
3.3. Reactive Oxygen Species (ROS)
3.4. Indoleamine-2,3-Dioxygenase (IDO)
3.5. PD-L1
3.6. IL-10
4. Histologic Localization to Elucidate MDSCs Function
5. Inducers of MDSCs
5.1. Induction Regimens
5.2. Medications
5.2.1. Steroids
5.2.2. mTOR Inhibitor: Rapamycin
5.2.3. Calcineurin Inhibitor: Cyclosporine
5.2.4. IL-6
5.2.5. IL-33
5.2.6. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (Aryl Hydrocarbon Receptor Agonist)
5.2.7. Hepatic Stellate Cells
6. Inhibitors of MDSCs
6.1. All-Trans Retinoic Acid (ATRA)
6.2. MEK Inhibitor
6.3. Anti-PD-L1 Antibodies
6.4. IDO Inhibitors
7. Isolation Techniques
8. Relationships between MDSCs and Other Immune Cells
8.1. MDSCs and Tregs
8.2. MDSCs, Dendritic Cells, and Macrophages
8.3. MDSCs and Bregs
8.4. MDSCs and NKT Cells
8.5. MDSCs and γδ T Cells
9. Clinical Organ Transplantation
10. Feature Perspective and Possible Clinical Application
10.1. Cell. Therapy
10.2. Tolerance Induction “Integration MDSCs and Other Immune Cells in Organ Transplantation”
10.3. Marker of Immunosuppression
10.4. Source for MDSCs
11. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| MDSCs | myeloid-derived suppressor cells |
| G-MDSCs | granulocytic MDSCs |
| M-MDSCs | monocytic MDSCs |
| e-MDSCs | early stage MDSCs |
| iNOS | inducible NO synthase |
| ROS | reactive Oxygen Species |
| PBMC | peripheral blood mononuclear cell |
| IDO | indoleamine-2,3-dioxygenase |
| DCs | dendritic cells |
| ECDI | 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide |
| ECDI-SP | donor splenocytes treated with 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide |
| STAT | signal transducers and activators of transcription |
| CSF | colony stimulating factor |
| TCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
| AhR | aryl hydrocarbon receptor |
| HSCs | Hepatic stellate cells |
| ATRA | All-trans retinoic acid |
| MACS | magnetic-activated cell sorting |
| FACS | flow cytometric cell sorter |
| DCregs | regulatory DCs |
| Mregs | regulatory macrophages |
| NKT cells | Natural Killer T cells |
| iNKT cells | invariant NKT cells |
References
- Young, M.R.; Newby, M.; Wepsic, H.T. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987, 47, 100–105. [Google Scholar] [PubMed]
- Pak, A.S.; Wright, M.A.; Matthews, J.P.; Collins, S.L.; Petruzzelli, G.J.; Young, M.R. Mechanisms of immune suppression in patients with head and neck cancer: Presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res. 1995, 1, 95–103. [Google Scholar] [PubMed]
- Yang, R.; Cai, Z.; Zhang, Y.; Yutzy, W.H.; Roby, K.F.; Roden, R.B. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006, 66, 6807–6815. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pan, P.Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Dugast, A.S.; Haudebourg, T.; Coulon, F.; Heslan, M.; Haspot, F.; Poirier, N.; Vuillefroy de Silly, R.; Usal, C.; Smit, H.; Martinet, B.; et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J. Immunol. 2008, 180, 7898–7906. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.R.; Ledgerwood, L.; Yang, Y.; Xu, J.; Lal, G.; Burrell, B.; Ma, G.; Hashimoto, D.; Li, Y.; Boros, P.; et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J. Clin. Investig. 2010, 120, 2486–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, Y.; Qin, J.; Chou, H.S.; Bhatt, S.; Wang, L.; Stuehr, D.; Ghosh, A.; Fung, J.J.; Lu, L.; Qian, S. Cotransplantation with myeloid-derived suppressor cells protects cell transplants: A crucial role of inducible nitric oxide synthase. Transplantation 2014, 97, 740–747. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Trino, S.; Laurenzana, I.; Lamorte, D.; Caivano, A.; Del Vecchio, L.; Musto, P. Mesenchymal Stem Cell Derived Extracellular Vesicles: A Role in Hematopoietic Transplantation? Int. J. Mol. Sci. 2017, 18, 1022. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Mosheir, E.; Menon, M.C.; Wilson, D.; Woytovich, C.; Ochando, J.; Murphy, B. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am. J. Transplant. 2013, 13, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nakao, T.; Yoshimura, N.; Ashihara, E. Rapamycin Prolongs Cardiac Allograft Survival in a Mouse Model by Inducing Myeloid-Derived Suppressor Cells. Am. J. Transplant. 2015, 15, 2364–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Liang, S.; Wu, J.; Horuzsko, A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation 2008, 86, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, H.R.; Zhao, Z.; Rosborough, B.R.; Liu, Q.; Castellaneta, A.; Isse, K.; Wang, Z.; Lang, M.; Stolz, D.B.; Zheng, X.X. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T. cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J. Immunol. 2011, 187, 4598–4610. [Google Scholar] [CrossRef] [PubMed]
- Adeegbe, D.; Serafini, P.; Bronte, V.; Zoso, A.; Ricordi, C.; Inverardi, L. In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 2011, 20, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kheradmand, T.; Bryant, J.; Wang, S.; Tasch, J.; Wang, J.J.; Zhang, Z.; Luo, X. Intragraft CD11b(+) IDO(+) cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am. J. Transplant. 2012, 12, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Dilek, N.; Poirier, N.; Usal, C.; Martinet, B.; Blancho, G.; Vanhove, B. Control of transplant tolerance and intragraft regulatory T cell localization by myeloid-derived suppressor cells and CCL5. J. Immunol. 2012, 188, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Hongo, D.; Tang, X.; Baker, J.; Engleman, E.G.; Strober, S. Requirement for interactions of natural killer T cells and myeloid-derived suppressor cells for transplantation tolerance. Am. J. Transplant. 2014, 14, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Lerret, N.M.; Wang, J.J.; Kang, H.K.; Tasch, J.; Zhang, Z.; Luo, X. Preemptive donor apoptotic cell infusions induce IFN-gamma-producing myeloid-derived suppressor cells for cardiac allograft protection. J. Immunol. 2014, 192, 6092–6101. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, X.; Bi, Y.; Shen, B.; Shao, K.; Yang, H.; Lu, Y.; Zhang, Z.; Chen, X.; Liu, H.; et al. Dexamethasone potentiates myeloid-derived suppressor cell function in prolonging allograft survival through nitric oxide. J. Leukoc. Biol. 2014, 96, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajardo, T.; Morales, R.A.; Campos-Mora, M.; Campos-Acuna, J.; Pino-Lagos, K. Exogenous interleukin-33 targets myeloid-derived suppressor cells and generates periphery-induced Foxp3(+) regulatory T cells in skin-transplanted mice. Immunology 2015, 146, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Delta(9)-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nakao, T.; Ashihara, E.; Yoshimura, N. Myeloid-derived Suppressor Cells Recruit CD4(+)/Foxp3(+) Regulatory T Cells in a Murine Cardiac Allograft. Transplant. Proc. 2016, 48, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Y.; Wu, T.; Na, N.; Zhao, Y.; Li, W.; Han, C.; Zhang, L.; Lu, J.; Zhao, Y. TNFalpha-induced M-MDSCs promote transplant immune tolerance via nitric oxide. J. Mol. Med. 2016, 94, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, X.F.; Cao, K.; Ding, J.; Kang, X.; Guan, W.X.; Ding, Y.T.; Liu, B.R.; Du, J.F. Dexamethasone-Induced Myeloid-Derived Suppressor Cells Prolong Allo Cardiac Graft Survival through iNOS- and Glucocorticoid Receptor-Dependent Mechanism. Front. Immunol. 2018, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Nakamura, T.; Masuda, K.; Matsuyama, T.; Ushigome, H.; Ashihara, E.; Yoshimura, N. Dexamethasone Prolongs Cardiac Allograft Survival in a Murine Model Through Myeloid-derived Suppressor Cells. Transplant. Proc. 2018, 50, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Zahorchak, A.F.; Ezzelarab, M.B.; Lu, L.; Turnquist, H.R.; Thomson, A.W. In Vivo Mobilization and Functional Characterization of Nonhuman Primate Monocytic Myeloid-Derived Suppressor Cells. Am. J. Transplant. 2016, 16, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Chen, S.; Guo, X.; Chen, Z.; Huang, X.; Lai, Y.; Lin, M. Clinical significance of myeloid-derived suppressor cells in human renal transplantation with acute T cell-mediated rejection. Inflammation 2014, 37, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Hock, B.D.; McKenzie, J.L.; Cross, N.B.; Currie, M.J. Dynamic changes in myeloid derived suppressor cell subsets following renal transplant: A. prospective study. Transpl. Immunol. 2015, 32, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Rekers, N.V.; Bajema, I.M.; Mallat, M.J.; Petersen, B.; Anholts, J.D.; Swings, G.M.; van Miert, P.P.; Kerkhoff, C.; Roth, J.; Popp, D.; et al. Beneficial Immune Effects of Myeloid-Related Proteins in Kidney Transplant Rejection. Am. J. Transplant. 2016, 16, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Abu-Elmagd, K.; Kish, D.D.; Keslar, K.; Baldwin, W.M., 3rd; Fairchild, R.L.; Fujiki, M.; Khanna, A.; Osman, M.; Costa, G.; et al. Myeloid-derived suppressor cells increase and inhibit donor-reactive T cell responses to graft intestinal epithelium in intestinal transplant patients. Am. J. Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J.; Ruscetti, M.; Arenzana, T.L.; Tran, L.M.; Bianci-Frias, D.; Sybert, E.; Priceman, S.J.; Wu, L.; Nelson, P.S.; Smale, S.T.; et al. Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol. Cell. Biol. 2014, 34, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Fujii, W.; Ashihara, E.; Hirai, H.; Nagahara, H.; Kajitani, N.; Fujioka, K.; Murakami, K.; Seno, T.; Yamamoto, A.; Ishino, H.; et al. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J. Immunol. 2013, 191, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daley, J.M.; Thomay, A.A.; Connolly, M.D.; Reichner, J.S.; Albina, J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008, 83, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haile, L.A.; Gamrekelashvili, J.; Manns, M.P.; Korangy, F.; Greten, T.F. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol. 2010, 185, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Prins, R.M.; Scott, G.P.; Merchant, R.E.; Graf, M.R. Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer. Immunol. Immunother. 2002, 51, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Jitschin, R.; Braun, M.; Buttner, M.; Dettmer-Wilde, K.; Bricks, J.; Berger, J.; Eckart, M.J.; Krause, S.W.; Oefner, P.J.; Le Blanc, K.; et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014, 124, 750–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasquez-Dunddel, D.; Pan, F.; Zeng, Q.; Gorbounov, M.; Albesiano, E.; Fu, J.; Blosser, R.L.; Tam, A.J.; Bruno, T.; Zhang, H.; et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Investig. 2013, 123, 1580–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, P.C.; Sharma, P.; Kapasi, A.A.; Reddy, K.; Franki, N.; Gibbons, N. Morphine enhances macrophage apoptosis. J. Immunol. 1998, 160, 1886–1893. [Google Scholar] [PubMed]
- Lopes, M.F.; da Veiga, V.F.; Santos, A.R.; Fonseca, M.E.; DosReis, G.A. Activation-induced CD4+ T cell death by apoptosis in experimental Chagas’ disease. J. Immunol. 1995, 154, 744–752. [Google Scholar] [PubMed]
- Koblish, H.K.; Hunter, C.A.; Wysocka, M.; Trinchieri, G.; Lee, W.M. Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: Inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J. Exp. Med. 1998, 188, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Harari, O.; Liao, J.K. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr. Pharm. Des. 2004, 10, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Serafini, P.; De Santo, C.; Marigo, I.; Tosello, V.; Mazzoni, A.; Segal, D.M.; Staib, C.; Lowel, M.; Sutter, G.; et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 2003, 170, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Hernandez, C.P.; Quiceno, D.; Dubinett, S.M.; Zabaleta, J.; Ochoa, J.B.; Gilbert, J.; Ochoa, A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005, 202, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelp, M.T.; Kates, P.A.; Hunt, J.T.; Newitt, J.A.; Balog, A.; Maley, D.; Zhu, X.; Abell, L.; Allentoff, A.; Borzilleri, R.; et al. Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc. Natl. Acad. Sci. USA. 2018, 115, 3249–3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mougiakakos, D.; Jitschin, R.; von Bahr, L.; Poschke, I.; Gary, R.; Sundberg, B.; Gerbitz, A.; Ljungman, P.; Le Blanc, K. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia 2013, 27, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Eguchi, H.; Nakahata, K.; Lo, P.C.; Yamanaka, K.; Kawamura, T.; Matsuura, R.; Sakai, R.; Asada, M.; Okuyama, H.; et al. Monocytic MDSCs regulate macrophage-mediated xenogenic cytotoxicity. Transpl. Immunol. 2015, 33, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bi, Y.; Xue, L.; Liao, J.; Chen, X.; Lu, Y.; Zhang, Z.; Wang, J.; Liu, H.; Yang, H.; et al. The calcineurin-NFAT axis controls allograft immunity in myeloid-derived suppressor cells through reprogramming T cell differentiation. Mol. Cell. Biol. 2015, 35, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D.; et al. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smahel, M. PD-1/PD-L1 Blockade Therapy for Tumors with Downregulated MHC Class, I. Expression. Int. J. Mol. Sci. 2017, 18, 1331. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Ballbach, M.; Dannert, A.; Singh, A.; Siegmund, D.M.; Handgretinger, R.; Piali, L.; Rieber, N.; Hartl, D. Expression of checkpoint molecules on myeloid-derived suppressor cells. Immunol. Lett. 2017, 192, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Demirci, G.; Strom, T.B.; Li, X.C. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation 2003, 76, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T. regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ligocki, A.J.; Niederkorn, J.Y. Advances on Non-CD4 + Foxp3+ T Regulatory Cells: CD8+, Type 1, and Double Negative T Regulatory Cells in Organ Transplantation. Transplantation 2015, 99, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Wortel, C.M.; Heidt, S.; Regulatory, B. cells: Phenotype, function and role in transplantation. Transplant. Immunol. 2017, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Lee, S.H.; Kim, E.K.; Lee, E.J.; Baek, J.A.; Park, S.H.; Kwok, S.K.; Cho, M.L. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci. Rep. 2018, 8, 3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronte, V.; Chappell, D.B.; Apolloni, E.; Cabrelle, A.; Wang, M.; Hwu, P.; Restifo, N.P. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J. Immunol. 1999, 162, 5728–5737. [Google Scholar] [PubMed]
- Park, M.J.; Lee, S.H.; Kim, E.K.; Lee, E.J.; Park, S.H.; Kwok, S.K.; Cho, M.L. Myeloid-Derived Suppressor Cells Induce the Expansion of Regulatory B Cells and Ameliorate Autoimmunity in the Sanroque Mouse Model of Systemic Lupus Erythematosus. Arthritis Rheum. 2016, 68, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Joyce, D.; Fujino, M.; Morita, M.; Araki, R.; Fung, J.; Qian, S.; Lu, L.; Li, X.K. Induced pluripotent stem cells-derived myeloid-derived suppressor cells regulate the CD8(+) T. cell response. Stem Cell Res. 2018, 29, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, W.; Cho, S.K.; Park, S.G. Characterization of Multiple Cytokine Combinations and TGF-beta on Differentiation and Functions of Myeloid-Derived Suppressor Cells. Int. J. Mol. Sci. 2018, 19, 869. [Google Scholar] [CrossRef]
- Ungefroren, H.; Witte, D.; Fiedler, C.; Gadeken, T.; Kaufmann, R.; Lehnert, H.; Gieseler, F.; Rauch, B.H. The Role of PAR2 in TGF-beta1-Induced ERK Activation and Cell Motility. Int. J. Mol. Sci. 2017, 18, 2776. [Google Scholar] [CrossRef]
- Guidez, F.; Li, A.C.; Horvai, A.; Welch, J.S.; Glass, C.K. Differential utilization of Ras signaling pathways by macrophage colony-stimulating factor (CSF) and granulocyte-macrophage CSF receptors during macrophage differentiation. Mol. Cell. Biol. 1998, 18, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, M.; Masuda, S. Role of mTOR Inhibitors in Kidney Disease. Int. J. Mol. Sci. 2016, 17, 975. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, B.; Shan, W.; Tan, Y.; Feng, J.; Xu, L.; Wang, L.; Han, B.; Zhang, M.; Yu, J. mTOR inhibitor rapamycin induce polymorphonuclear myeloid-derived suppressor cells mobilization and function in protecting against acute graft-versus-host disease after bone marrow transplantation. Clin. Immunol. 2018, 187, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, Y.; Yang, H.; Chen, X.; Liu, H.; Lu, Y.; Zhang, Z.; Liao, J.; Yang, S.; Chu, Y.; et al. mTOR limits the recruitment of CD11b+Gr1+Ly6Chigh myeloid-derived suppressor cells in protecting against murine immunological hepatic injury. J. Leukocyte Biol. 2014, 95, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Ma, L.; Teruya-Feldstein, J.; Rojo, F.; Salmena, L.; Alimonti, A.; Egia, A.; Sasaki, A.T.; Thomas, G.; Kozma, S.C.; et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Investig. 2008, 118, 3065–3074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Zhao, Y.; Wang, H.; Li, Y.; Shao, L.; Wang, R.; Lu, J.; Yang, Z.; Wang, J.; Zhao, Y. mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci. Rep. 2016, 6, 20250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Kishimoto, T. Interleukin 6 and plasma cell neoplasias. Prog. Growth Factor Res. 1989, 1, 133–142. [Google Scholar] [CrossRef]
- Bunt, S.K.; Yang, L.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007, 67, 10019–10026. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Oughton, J.A.; Kerkvliet, N.I. Functional alterations in CD11b(+)Gr-1(+) cells in mice injected with allogeneic tumor cells and treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Int. Immunopharmacol. 2003, 3, 553–570. [Google Scholar] [CrossRef]
- Goudot, C.; Coillard, A.; Villani, A.C.; Gueguen, P.; Cros, A.; Sarkizova, S.; Tang-Huau, T.L.; Bohec, M.; Baulande, S.; Hacohen, N.; et al. Aryl Hydrocarbon Receptor Controls Monocyte Differentiation into Dendritic Cells versus Macrophages. Immunity 2017, 47, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Nakahara, T.; Hashimoto-Hachiya, A.; Furue, M.; Tsuji, G. Glyteer, Soybean Tar, Impairs IL-4/Stat6 Signaling in Murine Bone Marrow-Derived Dendritic Cells: The Basis of Its Therapeutic Effect on Atopic Dermatitis. Int. J. Mol. Sci. 2018, 19, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Voorhis, M.; Fechner, J.H.; Zhang, X.; Mezrich, J.D. The aryl hydrocarbon receptor: A novel target for immunomodulation in organ transplantation. Transplantation 2013, 95, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Bohar, Z.; Toldi, J.; Fulop, F.; Vecsei, L. Changing the face of kynurenines and neurotoxicity: Therapeutic considerations. Int. J. Mol. Sci. 2015, 16, 9772–9793. [Google Scholar] [CrossRef] [PubMed]
- Maliniemi, P.; Laukkanen, K.; Vakeva, L.; Dettmer, K.; Lipsanen, T.; Jeskanen, L.; Bessede, A.; Oefner, P.J.; Kadin, M.E.; Ranki, A.; et al. Biological and clinical significance of tryptophan-catabolizing enzymes in cutaneous T.-cell lymphomas. Oncoimmunology 2017, 6, e1273310. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Investig. 2013, 123, 1902–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochst, B.; Schildberg, F.A.; Sauerborn, P.; Gabel, Y.A.; Gevensleben, H.; Goltz, D.; Heukamp, L.C.; Turler, A.; Ballmaier, M.; Gieseke, F.; et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J. Hepatol. 2013, 59, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Perez-Gutierrez, A.; Yovchev, M.I.; Matsubara, K.; Yokota, S.; Guzman-Lepe, J.; Handa, K.; Collin de l’Hortet, A.; Thomson, A.W.; Geller, D.; et al. Regeneration and Cell Recruitment in an Improved Heterotopic Auxiliary Partial Liver Transplantation Model in the Rat. Transplantation 2017, 101, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, R.; Udonta, F.; Wroblewski, M.; Ben-Batalla, I.; Santos, I.M.; Taverna, F.; Kuhlencord, M.; Gensch, V.; Pasler, S.; Vinckier, S.; et al. Blockade of myeloid-derived suppressor cell expansion with all-trans retinoic acid increases the efficacy of anti-angiogenic therapy. Cancer Res. 2018, 78, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Highfill, S.L.; Cui, Y.; Smith, J.P.; Walker, A.J.; Ramakrishna, S.; El-Etriby, R.; Galli, S.; Tsokos, M.G.; Orentas, R.J.; et al. Reduction of MDSCs with All-trans Retinoic Acid Improves CAR Therapy Efficacy for Sarcomas. Cancer Immunol. Res. 2016, 4, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nefedova, Y.; Fishman, M.; Sherman, S.; Wang, X.; Beg, A.A.; Gabrilovich, D.I. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007, 67, 11021–11028. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kakegawa, J.; Yamaguchi, T.; Hantani, Y.; Okajima, N.; Sakai, T.; Watanabe, Y.; Nakamura, M. Identification and characterization of a novel chemotype MEK inhibitor able to alter the phosphorylation state of MEK1/2. Oncotarget 2012, 3, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kakefuda, R.; Tajima, N.; Sowa, Y.; Sakai, T. Antitumor activities of JTP-74057 (GSK1120212), a novel MEK1/2 inhibitor, on colorectal cancer cell lines in vitro and in vivo. Int. J. Oncol. 2011, 39, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Allegrezza, M.J.; Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Perales-Puchalt, A.; Nguyen, J.M.; Payne, K.K.; Singhal, S.; Eruslanov, E.B.; Tchou, J.; et al. Trametinib Drives T-cell-Dependent Control of KRAS-Mutated Tumors by Inhibiting Pathological Myelopoiesis. Cancer Res. 2016, 76, 6253–6265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torphy, R.J.; Schulick, R.D.; Zhu, Y. Newly Emerging Immune Checkpoints: Promises for Future Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2642. [Google Scholar] [CrossRef] [PubMed]
- Young, J.S.; Daniels, M.D.; Miller, M.L.; Wang, T.; Zhong, R.; Yin, D.; Alegre, M.L.; Chong, A.S. Erosion of Transplantation Tolerance After Infection. Am. J. Transplant. 2017, 17, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Barsoumian, H.B.; Schoenhals, J.E.; Cushman, T.R.; Caetano, M.S.; Wang, X.; Valdecanas, D.R.; Niknam, S.; Younes, A.I.; Li, G.; et al. Indoleamine 2,3-dioxygenase 1 inhibition targets anti-PD1-resistant lung tumors by blocking myeloid-derived suppressor cells. Cancer Lett. 2018, 431, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, X.; Liu, Z.; Xu, H.; Wang, T.; He, L.; Zhao, A.; et al. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF-beta/beta-catenin pathway. Mol. Hum. Reprod. 2016, 22, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Hoechst, B.; Gamrekelashvili, J.; Manns, M.P.; Greten, T.F.; Korangy, F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 2011, 117, 6532–6541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.R.; Kwak, Y.; Yang, T.; Han, J.H.; Park, S.H.; Ye, M.B.; Lee, W.; Sim, K.Y.; Kang, J.A.; Kim, Y.C.; et al. Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-beta during Murine Colitis. Cell Rep. 2016, 17, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, M.K.; Moore, D.J.; Sonawane, S.B.; Duff, P.E.; O’Connor, M.R.; Yeh, H.; Lian, M.M.; Deng, S.; Caton, A.J.; et al. Regulatory T-cell counter-regulation by innate immunity is a barrier to transplantation tolerance. Am. J. Transplant. 2009, 9, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Todo, S.; Yamashita, K.; Goto, R.; Zaitsu, M.; Nagatsu, A.; Oura, T.; Watanabe, M.; Aoyagi, T.; Suzuki, T.; Shimamura, T.; et al. A Pilot Study of Operational Tolerance with a Regulatory T Cell-Based Cell Therapy in Living Donor Liver Transplantation. Hepatology 2016, 64, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Suri, R.M.; Niimi, M.; Ogilvie, A.L.; Kukutsch, N.A.; Rossner, S.; Schuler, G.; Austyn, J.M.; et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur. J. Immunol. 2000, 30, 1813–1822. [Google Scholar] [CrossRef]
- Baas, M.C.; Kuhn, C.; Valette, F.; Mangez, C.; Duarte, M.S.; Hill, M.; Besancon, A.; Chatenoud, L.; Cuturi, M.C.; You, S. Combining autologous dendritic cell therapy with CD3 antibodies promotes regulatory T cells and permanent islet allograft acceptance. J. Immunol. 2014, 193, 4696–4703. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, P.; Tomiuk, S.; Kammler, A.; Fandrich, F.; Schlitt, H.J.; Geissler, E.K.; Hutchinson, J.A. IFN-gamma-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol. Ther. 2013, 21, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Carretero-Iglesia, L.; Bouchet-Delbos, L.; Louvet, C.; Drujont, L.; Segovia, M.; Merieau, E.; Chiffoleau, E.; Josien, R.; Hill, M.; Cuturi, M.C.; et al. Comparative Study of the Immunoregulatory Capacity of In Vitro Generated Tolerogenic Dendritic Cells, Suppressor Macrophages, and Myeloid-Derived Suppressor Cells. Transplantation 2016, 100, 2079–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahorchak, A.F.; Macedo, C.; Hamm, D.E.; Butterfield, L.H.; Metes, D.M.; Thomson, A.W. High PD-L1/CD86 MFI ratio and IL-10 secretion characterize human regulatory dendritic cells generated for clinical testing in organ transplantation. Cell. Immunol. 2018, 323, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, P.; Haarer, J.; Kammler, A.; Walter, L.; Tomiuk, S.; Ahrens, N.; Wege, A.K.; Goecze, I.; Zecher, D.; Banas, B.; et al. TIGIT(+) iTregs elicited by human regulatory macrophages control T. cell immunity. Nat. Commun. 2018, 9, 2858. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, Y.; Zheng, D.; Sun, Y.; Wang, Y.; Lee, V.W.; Zhang, G.; Tan, T.K.; Ince, J.; Alexander, S.I.; et al. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J. Am. Soc. Nephrol. 2010, 21, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, H.R.; Raimondi, G.; Zahorchak, A.F.; Fischer, R.T.; Wang, Z.; Thomson, A.W. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T. cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 2007, 178, 7018–7031. [Google Scholar] [CrossRef] [PubMed]
- Taner, T.; Hackstein, H.; Wang, Z.; Morelli, A.E.; Thomson, A.W. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am. J. Transplant. 2005, 5, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, B.; Lim, H.; Park, S.G. Immunomodulatory Function of Myeloid-Derived Suppressor Cells during B Cell-Mediated Immune Responses. Int. J. Mol. Sci. 2018, 19, 1468. [Google Scholar] [CrossRef]
- Katz, S.I.; Parker, D.; Turk, J.L. B-cell suppression of delayed hypersensitivity reactions. Nature 1974, 251, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Yu, Q.; Zheng, S.; Jiang, Y.; Liu, Y.; Yuan, G.; Qiu, L. Aberrant frequency of IL-10-producing B cells and its association with Treg and MDSC cells in Non Small Cell Lung Carcinoma patients. Hum. Immunol. 2016, 77, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Richt, J.A.; Driver, J.P. Harnessing Invariant NKT Cells to Improve Influenza Vaccines: A. Pig Perspective. Int. J. Mol. Sci. 2017, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Mussai, F.; De Santo, C.; Cerundolo, V. Interaction between invariant NKT cells and myeloid-derived suppressor cells in cancer patients: Evidence and therapeutic opportunities. J. Immunother. 2012, 35, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sachs, D.H.; Sykes, M.; Cosimi, A.B. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2013, 368, 1850–1852. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Aoyama, A.; Oura, T.; Yamada, Y.; Tonsho, M.; Huh, K.H.; Kawai, K.; Schoenfeld, D.; Allan, J.S.; Madsen, J.C.; et al. Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI Insight 2016, 1, e86419. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Oura, T.; Dehnadi, A.; Boskovic, S.; Matsunami, M.; Rosales, I.; Smith, R.N.; Colvin, R.B.; Cosimi, A.B.; Kawai, T.; et al. Long-term Nonhuman Primate Renal Allograft Survival Without Ongoing Immunosuppression in Recipients of Delayed Donor Bone Marrow Transplantation. Transplantation 2018, 102, e128–e136. [Google Scholar] [CrossRef] [PubMed]
- Ishii, R.; Hirai, T.; Miyairi, S.; Omoto, K.; Okumi, M.; Ishii, Y.; Tanabe, K.; et al. iNKT cell activation plus T-cell transfer establishes complete chimerism in a murine sublethal bone marrow transplant model. Am. J. Transplant. 2018, 18, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Sun, R.; Chen, Y.; Wei, H.; Tian, Z. gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J. Immunol. 2014, 193, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushigome, H.; Nakao, T.; Harada, S.; Koshino, K.; Suzuki, T.; Ito, T.; Nobori, S.; Yoshimura, N. Advantages and Disadvantages of Pre-emptive Kidney Transplantation: Results from a Single Transplantation Center. Transplant. Proc. 2015, 47, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Iida, T.; Ushigome, H.; Osaka, M.; Masuda, K.; Matsuyama, T.; Harada, S.; Nobori, S.; Yoshimura, N. Risk Factors and Management for Biliary Complications Following Adult Living-Donor Liver Transplantation. Ann. Transplant. 2017, 22, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.J.; Goto, R. Mechanisms of rejection: Current perspectives. Transplantation 2012, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Nakao, T.; Nakamura, T.; Harada, S.; Koshino, K.; Suzuki, T.; Ito, T.; Nobori, S.; Ushigome, H. Effectiveness of the Combination of Everolimus and Tacrolimus With High Dosage of Mizoribine for Living Donor-Related Kidney Transplantation. Transplant. Proc. 2016, 48, 786–789. [Google Scholar] [CrossRef] [PubMed]


| Author | Refs. | Year | Species | Organ/Tissue | Phenotype | Possible Mechanism of Suppression | CD4+ Tregs Involvement | Inducer | Remarkable Findings |
|---|---|---|---|---|---|---|---|---|---|
| Mouse/Rat | |||||||||
| Dugast | [5] | 2008 | Rat | Kidney | CD6−/NKRP−1+/CD80+/CD86+ | iNOS | + | anti CD28 Abs | Anti-CD28 Abs tolerance induction may dependent on iNOS+MDSCs. MDSC acted in a contact-dependent manner |
| Zhang | [11] | 2008 | Mouse | Skin | Gr-1+/CD11b+ | Arginase | N/A | ILT2 inhibitory receptor | Adoptive transfer of generated MDSCs prolonged skin allograft survival |
| Garcia | [6] | 2010 | Mouse | Heart | Gr-1+/CD11b+ | iNOS, Arginase | + | anti-CD40 Abs/DST | MDSCs migrated into the allograft prevent rejeciton and develop Tregs. Gr-1−/CD11b+ monocytes express PD-L1 |
| Turnquist | [12] | 2011 | Mouse | Heart | Gr-1int/CD11b+ | N/A | + | IL-33 | IL-33 induced MDSCs, but MDSCs did not prolong allograft survival in this model |
| Adeegbe | [13] | 2011 | Mouse | Skin | Gr-1+/CD11b+ | N/A | + | G-CSF, IL-2 | MDSCs and Tregs down-modulatd alloreactive T-cell responses in a synergistic manner |
| Chen | [14] | 2012 | Mouse | Heart | Gr-1+/CD11b+ | IDO | + | ECDI-SP | Allograft protection by ECDI-SP depended on MDSCs |
| Dilek | [15] | 2012 | Rat | Kidney | CD6−/NKRP-1+/CD80+/CD86+ | N/A | + | anti CD28 Abs | MDSCs contributed to the establishment of a graft to periphery CCL5 gradient |
| Arakawa | [7] | 2014 | Mouse | Islet | Gr-1+/CD11b+ | iNOS | N/A | GM-CSF, IL-4, hepatic stellate cells | In vitro generated MDSCs had an ability to protect allogeneic islet cells |
| Hongo | [16] | 2014 | Mouse | Heart | Gr-1+/CD11b+ | PDL1, arginase-1 | - | iNKT cells | mixed chimerism establishment required MDSCs |
| Bryant | [17] | 2014 | Mouse | Heart | Gr-1+/CD11b+ | IDO, iNOS | + | ECDI-SP | MDSCs protected allografts through their own production of IFN-γ |
| Liao | [18] | 2014 | Mouse | Skin | Gr-1+/CD11b+ | iNOS | N/A | dexamethasone | Glucocorticoid-glucocorticoid receptor-NO cascade was crucial by dexamethasone mediated immune suppression |
| Nakamura | [10] | 2015 | Mouse | Heart | Gr-1int/CD11b+ | iNOS | + | rapamycin | mTOR and Raf/MEK/ERK signaling pathways play an important role in MDSC expansion |
| Gajardo | [19] | 2015 | Mouse | Skin | Gr-1low/CD11b+ | iNOS, Arginase | + | IL-33 | IL-33 target cell population during transplant rejection corresponded to MDSCs |
| Sido | [20] | 2015 | Mouse | Skin | Gr-1+/CD11b+ | N/A | N/A | Delta(9)-Tetrahydrocannabinol | Delta(9)-Tetrahydrocannabinol induced MDSCs mainly through CB1 receptor |
| Nakamura | [21] | 2016 | Mouse | Heart | Gr-1+/CD11b+ | iNOS | + | rapamycin | MDSCs induced Tregs expansion in allografts |
| Yang | [22] | 2016 | Mouse | Skin | Gr-1+/CD11b+ | iNOS | N/A | M-CSF, TNFα | PD-L1 was upregulated on MDSCs |
| Zhao | [23] | 2018 | Mouse | Heart | Gr-1int/CD11b+ | iNOS | + | dexamethasone | GR signaling recruited transferred MDSCs into the allograft |
| Nakao | [24] | 2018 | Mouse | Heart | Gr-1+/CD11b+ | iNOS | + | dexamethasone | MDSCs regulated the expansion of Tregs |
| Other | |||||||||
| Zahorchak | [25] | 2015 | Macaque | N/A | CD33+/CD11b+/HLA-DR− | Arginase | + | GM-CSF, IL-4 | availability of cryopreserved MDSCs |
| Human | |||||||||
| Luan | [9] | 2013 | Human | Kidney | CD33+/CD11b+/HLA-DR− | N/A | + | N/A | There was a positive correlation between the number of MDSCs and Tregs |
| Meng | [26] | 2014 | Human | Kidney | CD33+/CD11b+/HLA-DR− | N/A | + | N/A | MDSCs associated with higher frequency of Tregs and better graft survival |
| Hock | [27] | 2015 | Human | Kidney | CD33+/CD45+/HLA-DR−/(CD14+/CD66b+) | N/A | N/A | N/A | The number of MDSCs increased following the initiation of immunosuppression. |
| Rekers | [28] | 2016 | Human | Kidney | CD33+/CD11b+/(CD14+) | ROS | + | S100A8, 9 | S100A9 expression predicted better graft outcomes |
| Okano | [29] | 2018 | Human | Intestine | CD33+/CD11b+/HLA-DR−/low | N/A | + | IL-6, exogenous steroid hormone | MDSCs in PBMC during rejection decreased |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T.; Ushigome, H. Myeloid-Derived Suppressor Cells as a Regulator of Immunity in Organ Transplantation. Int. J. Mol. Sci. 2018, 19, 2357. https://doi.org/10.3390/ijms19082357
Nakamura T, Ushigome H. Myeloid-Derived Suppressor Cells as a Regulator of Immunity in Organ Transplantation. International Journal of Molecular Sciences. 2018; 19(8):2357. https://doi.org/10.3390/ijms19082357
Chicago/Turabian StyleNakamura, Tsukasa, and Hidetaka Ushigome. 2018. "Myeloid-Derived Suppressor Cells as a Regulator of Immunity in Organ Transplantation" International Journal of Molecular Sciences 19, no. 8: 2357. https://doi.org/10.3390/ijms19082357