Responses of Transgenic Melatonin-Enriched Goats on LPS Stimulation and the Proteogenomic Profiles of Their PBMCs
Abstract
:1. Introduction
2. Results
2.1. Effects of Melatonin on Inflammatory Response Induced by LPS in Intact Goats
2.2. Proteogenomic Analysis of PBMCs
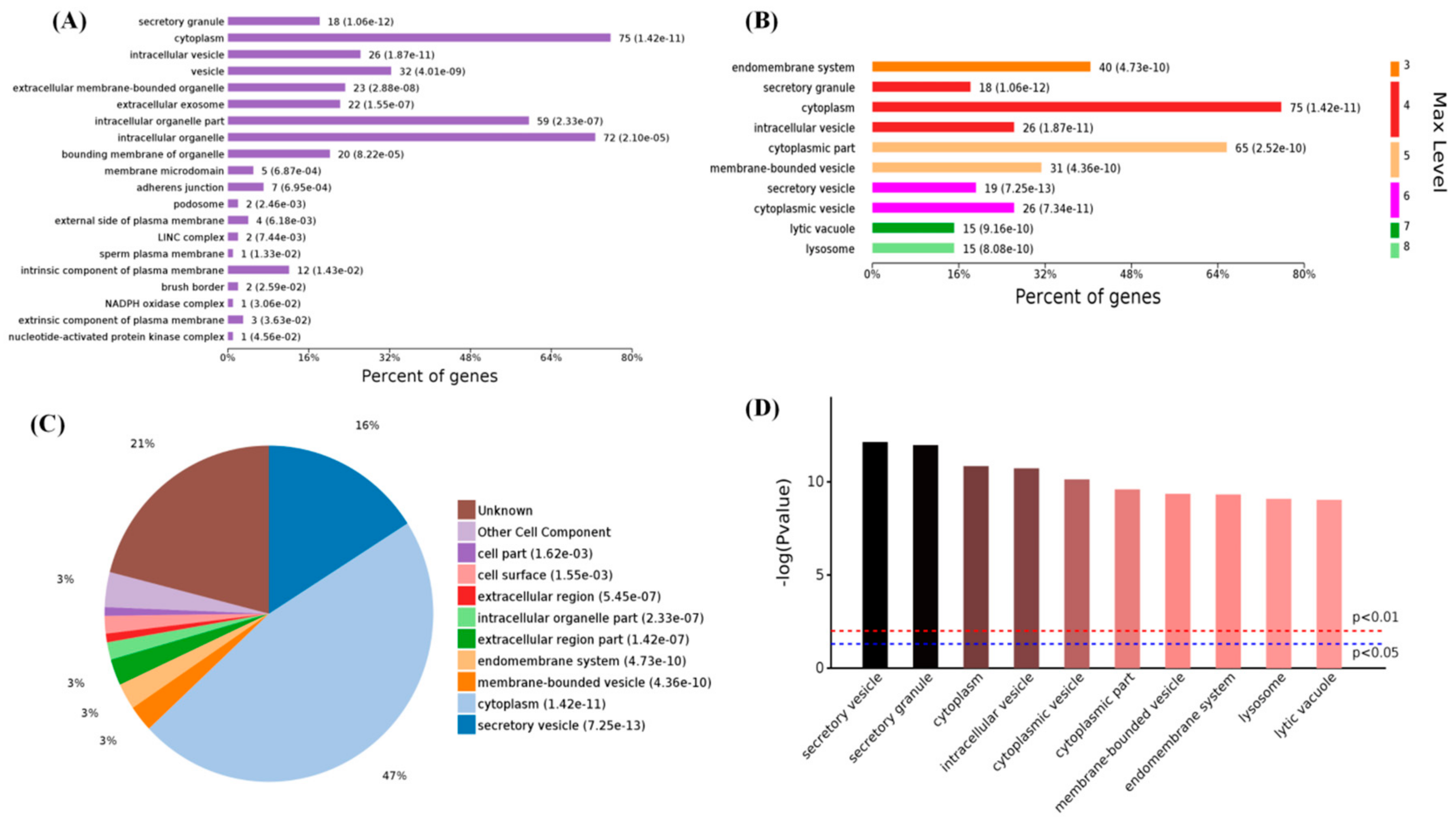
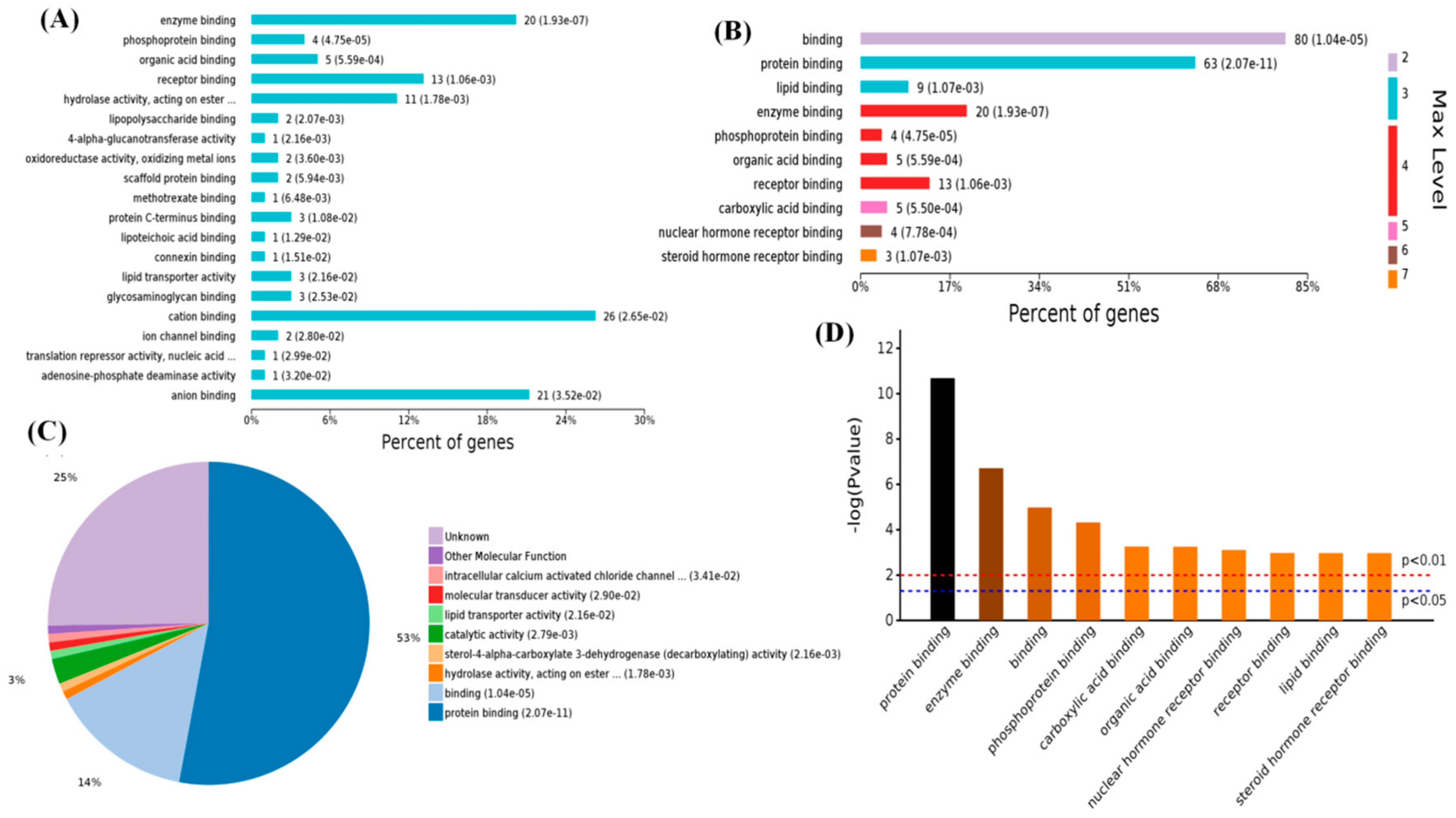
2.3. Functional Categorization of Overlapping Differential Proteins
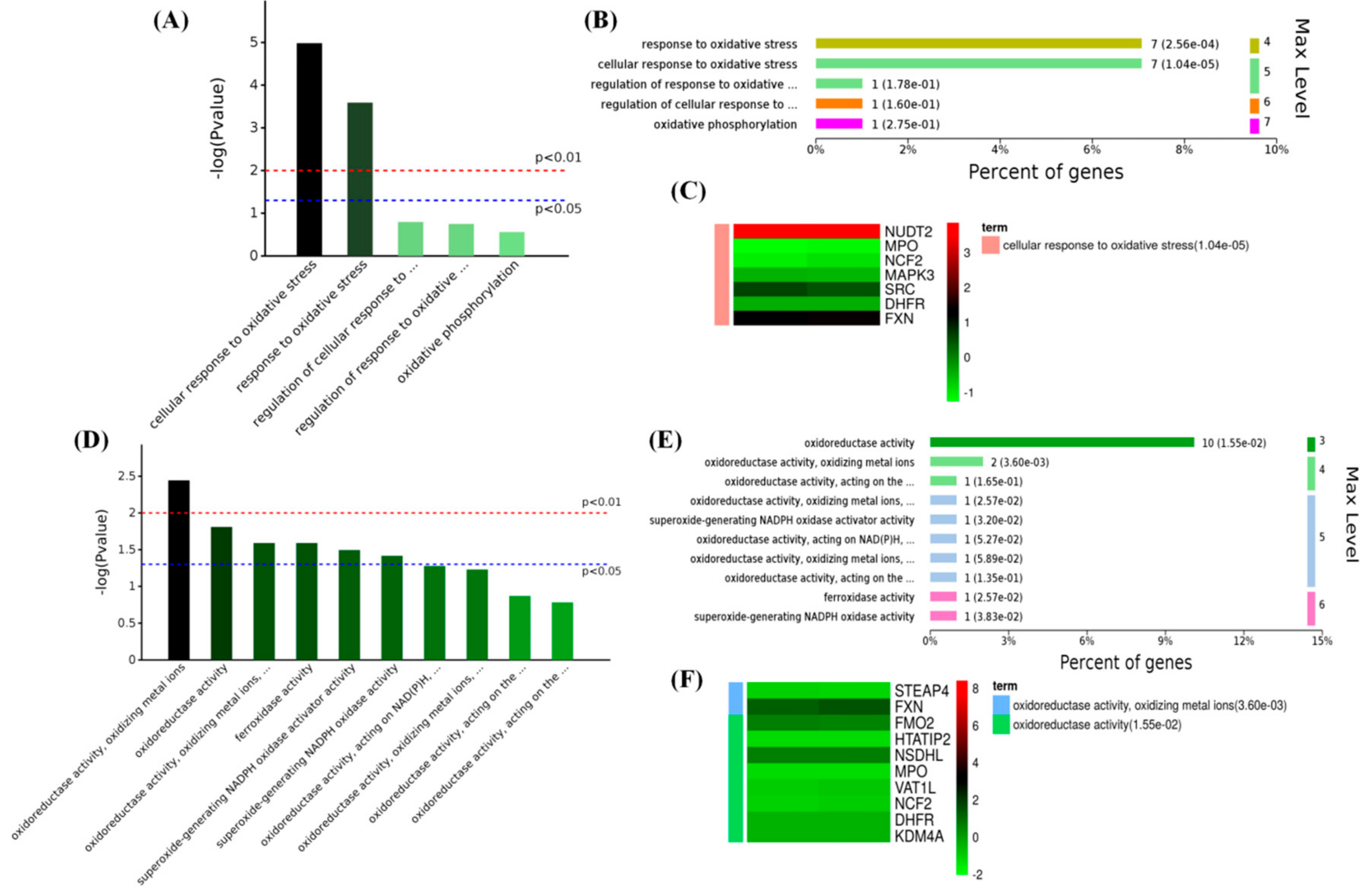
2.4. Melatonin Improved the Antioxidant Capacity in PBMCs
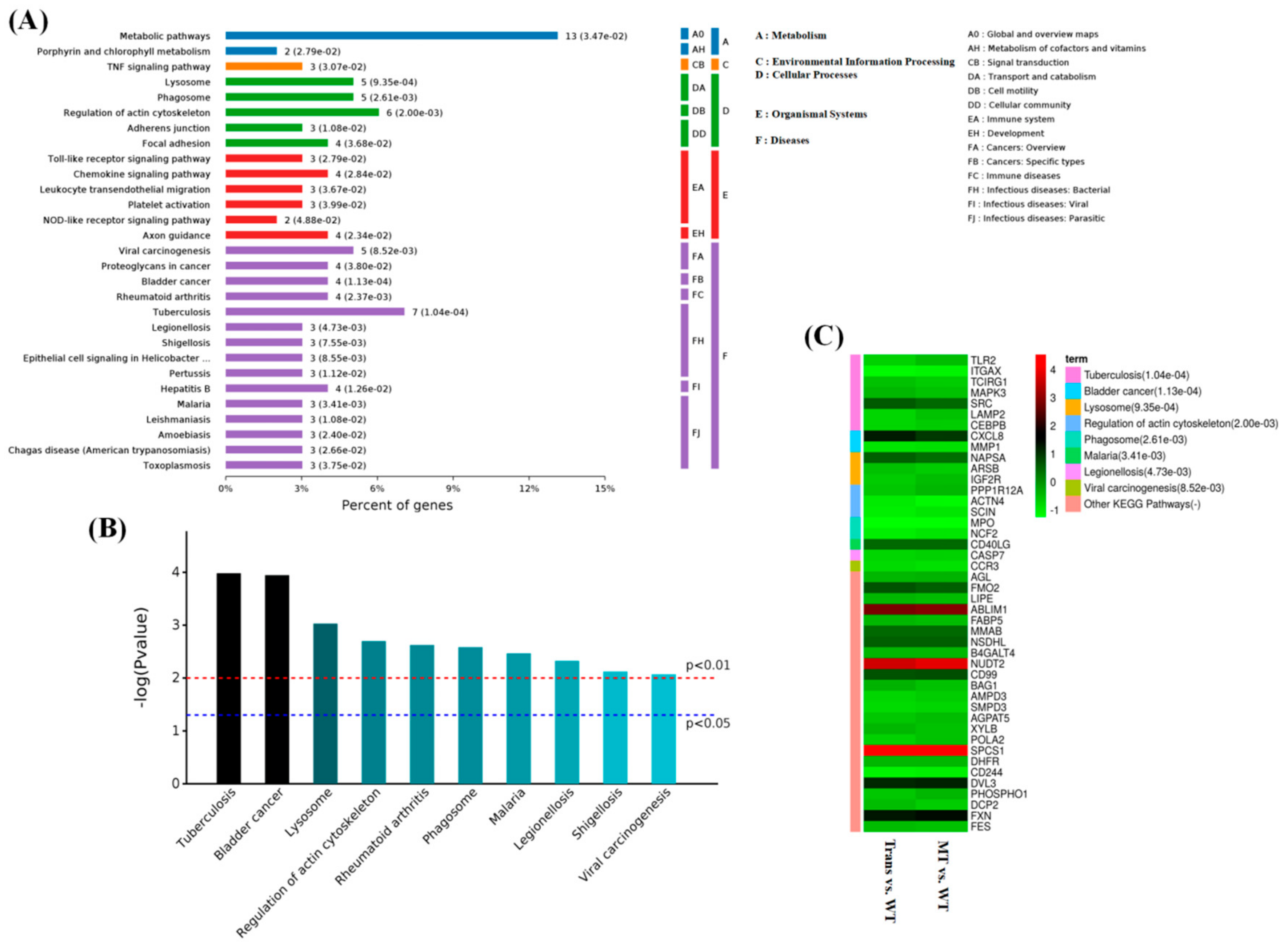
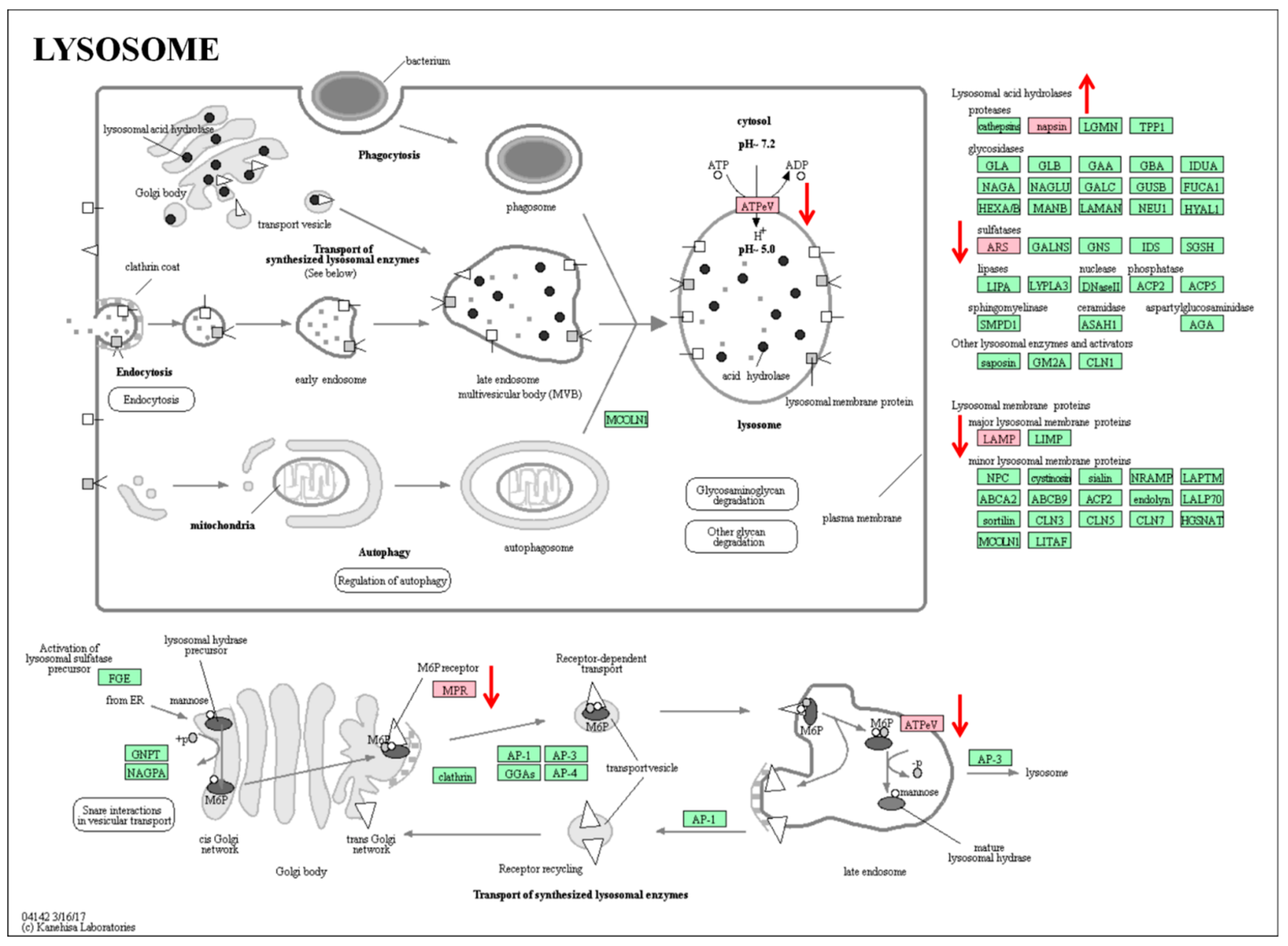
2.5. KEGG Pathway Analysis of Overlapping Differential Proteins
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Melatonin-Enriched Goat Model
4.3. In Vivo Study
4.4. Melatonin Assay
4.5. Detection of the Blood Physiological Index
4.6. PBMCs Isolation and Proteome Analysis
4.7. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Orhan, I.E.; Badiee, A.; Daglia, M.; Nabavi, S.M. Rhodiola rosea L. and Alzheimer′s Disease: From farm to pharmacy. Phytother. Res. 2016, 30, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Moore, X.L.; Dart, A.M.; Wang, L.M. Systemic inflammatory response following acute myocardial infarction. J. Geriatr. Cardiol. 2015, 12, 305–312. [Google Scholar] [PubMed]
- Hsu, C.C.; Lien, J.C.; Chang, C.W.; Chang, C.H.; Kuo, S.C.; Huang, T.F. Yuwen02f1 suppresses LPS-induced endotoxemia and adjuvant-induced arthritis primarily through blockade of ROS formation, NFkB and MAPK activation. Biochem. Pharmacol. 2013, 85, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Zingarelli, B.; Sheehan, M.; Wong, H.R. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit. Care Med. 2003, 31, S105–S111. [Google Scholar] [CrossRef] [PubMed]
- Rim, H.K.; Cho, W.; Sung, S.H.; Lee, K.T. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-kappaB pathways and protects mice from lethal endotoxin shock. J. Pharmacol. Exp. Ther. 2012, 342, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Luque, R.M.; Yubero-Serrano, E.M.; Cruz-Teno, C.; Ibanez-Costa, A.; Delgado-Lista, J.; Gracia-Navarro, F.; Perez-Jimenez, F.; Castano, J.P.; Lopez-Miranda, J. Dietary fat alters the expression of cortistatin and ghrelin systems in the PBMCs of elderly subjects: Putative implications in the postprandial inflammatory response. Mol. Nutr. Food Res. 2014, 58, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sai, J.; Xiong, L.; Zheng, J.; Liu, C.; Lu, Y.; Wang, G.; Wang, Y.; Wang, T.; Guan, X.; Chen, F.; et al. Protective Effect of Yinhua Miyanling Tablet on Lipopolysaccharide-Induced Inflammation through Suppression of NLRP3/Caspase-1 Inflammasome in Human Peripheral Blood Mononuclear Cells. Evid. Based Complement. Alternat. Med. 2016, 2016, 2758140. [Google Scholar] [CrossRef] [PubMed]
- Castellheim, A.; Brekke, O.L.; Espevik, T.; Harboe, M.; Mollnes, T.E. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand. J. Immunol. 2009, 69, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Cinar, M.U.; Islam, M.A.; Proll, M.; Kocamis, H.; Tholen, E.; Tesfaye, D.; Looft, C.; Schellander, K.; Uddin, M.J. Evaluation of suitable reference genes for gene expression studies in porcine PBMCs in response to LPS and LTA. BMC Res. Notes 2013, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Flori, L.; Lecardonnel, J.; Esquerre, D.; Hu, Z.L.; Teillaud, A.; Lemonnier, G.; Lefevre, F.; Oswald, I.P.; Rogel-Gaillard, C. Transcriptome analysis of porcine PBMCs after in vitro stimulation by LPS or PMA/ionomycin using an expression array targeting the pig immune response. BMC Genom. 2010, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Tzermpos, F.; Iatrou, I.; Papadimas, C.; Pistiki, A.; Georgitsi, M.; Giamarellos-Bourboulis, E.J. Function of blood monocytes among patients with orofacial infections. J. Craniomaxillofac. Surg. 2013, 41, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [PubMed]
- Finocchiaro, L.M.; Nahmod, V.E.; Launay, J.M. Melatonin biosynthesis and metabolism in peripheral blood mononuclear leucocytes. Biochem. J. 1991, 280, 727–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.T.; Ishizuka, B.; Kuribayashi, Y.; Amemiya, A.; Sumi, Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol. Hum. Reprod. 1999, 5, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Arto, M.; Hamilton, T.R.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Perez-Pe, R.; Muino-Blanco, T.; Cebrian-Perez, J.A.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin′s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, D.; Reiter, R.J.; Attia, A.M.; Hara, M.; Burgos, A.; Nistico, G. Potent protective effect of melatonin on in vivo paraquat-induced oxidative damage in rats. Life Sci. 1995, 56, 83–89. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Li, Y.; Zhu, K.; Xu, Z.; Song, Y.; Song, Y.; Liu, G. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 2015, 58, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Calvo, J.R.; Gonzalez-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Vinther, A.G.; Claesson, M.H. The influence of melatonin on the immune system and cancer. Ugeskr Laeger 2015, 177, V10140568. [Google Scholar] [CrossRef] [PubMed]
- D’Emmanuele Di Villa Bianca, R.; Marzocco, S.; di Paola, R.; Autore, G.; Pinto, A.; Cuzzocrea, S.; Sorrentino, R. Melatonin prevents lipopolysaccharide-induced hyporeactivity in rat. J. Pineal Res. 2004, 36, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Gilad, E.; Wong, H.R.; Zingarelli, B.; Virag, L.; O′Connor, M.; Salzman, A.L.; Szabo, C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: Role of inhibition of NFkappaB activation. FASEB J. 1998, 12, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Cavaillon, J.M. Septic shock. Lancet 2005, 365, 63–78. [Google Scholar] [CrossRef]
- Maestroni, G.J. Melatonin as a therapeutic agent in experimental endotoxic shock. J. Pineal Res. 1996, 20, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Requintina, P.J.; Oxenkrug, G.F. Differential effects of lipopolysaccharide on lipid peroxidation in F344N, SHR rats and BALB/c mice, and protection of melatonin and NAS against its toxicity. Ann. N. Y. Acad. Sci. 2003, 993, 325–333; discussion 345–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chiao, C.W.; Hsiao, G.; Chen, A.; Yen, M.H. Melatonin prevents endotoxin-induced circulatory failure in rats. J. Pineal Res. 2001, 30, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Crespo, E.; Macias, M.; Pozo, D.; Escames, G.; Martin, M.; Vives, F.; Guerrero, J.M.; Acuna-Castroviejo, D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999, 13, 1537–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escames, G.; Leon, J.; Macias, M.; Khaldy, H.; Acuna-Castroviejo, D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003, 17, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.Y.; Kim, B.S.; Kim, S.C.; Kim, D.H.; Chung, J.H. Microarray analysis of gene expression profiles in response to treatment with melatonin in lipopolysaccharide activated RAW 264.7 cells. Korean J. Physiol. Pharmacol. 2011, 15, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Sewerynek, E.; Melchiorri, D.; Reiter, R.J.; Ortiz, G.G.; Lewinski, A. Lipopolysaccharide-induced hepatotoxicity is inhibited by the antioxidant melatonin. Eur. J. Pharmacol. 1995, 293, 327–334. [Google Scholar] [CrossRef]
- Farez, M.F.; Mascanfroni, I.D.; Mendez-Huergo, S.P.; Yeste, A.; Murugaiyan, G.; Garo, L.P.; Balbuena Aguirre, M.E.; Patel, B.; Ysrraelit, M.C.; Zhu, C.; et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 2015, 162, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Messager, S.; Caillol, M.; Martinet, L. Long-term exposure of hypothalamic explants to melatonin alters the release of gonadotrophin releasing hormone and the density of melatonin binding sites in the pars tuberalis of the male mink (Mustela vison). J. Pineal Res. 1999, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Miller, S.C.; Osmond, D.G. Melatonin inhibits apoptosis during early B-cell development in mouse bone marrow. J. Pineal Res. 2000, 29, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.M.; Reiter, R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Calvo, J.R.; Karasek, M.; Guerrero, J.M. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J. Clin. Endocrinol. Metab. 2005, 90, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Pozo, D.; Garcia-Maurino, S.; Guerrero, J.M.; Calvo, J.R. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT, and hydroxindole-O-methyltransferase in different populations of human immune cells. J. Pineal Res. 2004, 37, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Garcia-Perganeda, A.; Naji, L.; Calvo, J.R.; Romero, M.P.; Guerrero, J.M. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell. Mol. Life Sci. 2003, 60, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Pozo, D.; Delgado, M.; Fernandez-Santos, J.M.; Calvo, J.R.; Gomariz, R.P.; Martin-Lacave, I.; Ortiz, G.G.; Guerrero, J.M. Expression of the Mel1a-melatonin receptor mRNA in T and B subsets of lymphocytes from rat thymus and spleen. FASEB J. 1997, 11, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Rodriguez, A.B.; Pariente, J.A. The inhibition of TNF-alpha-induced leucocyte apoptosis by melatonin involves membrane receptor MT1/MT2 interaction. J. Pineal Res. 2013, 54, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, C.P. Loss of intraventricular fluid melatonin can explain the neuropathology of Alzheimer’s disease. Med. Hypotheses 1997, 49, 153–158. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behav. Brain Funct. 2006, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Lowes, D.A.; Allen, L.; Cameron, G.; Aucott, L.S.; Webster, N.R. Melatonin as a potential therapy for sepsis: A phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J. Pineal Res. 2014, 56, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez Gonzalez, M.A.; Mata Maderuelo, F.; Delgado Moreno, F. Lymphocytes of patients with auto-immune deafness present type II collagen hyporeactivity in the presence of the pineal hormone melatonin. Acta Otorrinolaringol. Esp. 2000, 51, 314–318. [Google Scholar] [PubMed]
- Hu, Z.P.; Fang, X.L.; Fang, N.; Wang, X.B.; Qian, H.Y.; Cao, Z.; Cheng, Y.; Wang, B.N.; Wang, Y. Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF-kappaB system in high-fat-fed rabbits. J. Pineal Res. 2013, 55, 388–398. [Google Scholar] [PubMed]
- Alvarez-Sanchez, N.; Cruz-Chamorro, I.; Diaz-Sanchez, M.; Sarmiento-Soto, H.; Medrano-Campillo, P.; Martinez-Lopez, A.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Melatonin reduces inflammatory response in peripheral T helper lymphocytes from relapsing-remitting multiple sclerosis patients. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Saha, S.; Mahalanobish, S.; Sadhukhan, P.; Sil, P.C. Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. Food Chem. Toxicol. 2018, 118, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shi, J.; Tian, J.; Tao, J.; Chai, M.; Wang, J.; Xu, Z.; Song, Y.; Zhu, K.; Ji, P.; et al. Exogenous melatonin reduces somatic cell count of milk in holstein cows. Sci. Rep. 2017, 7, 43280. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Humar, B.; Gupta, A.; Maurizio, E.; Borgeaud, N.; Graf, R.; Clavien, P.A.; Tian, Y. Exogenous melatonin protects small-for-size liver grafts by promoting monocyte infiltration and releases interleukin-6. J. Pineal Res. 2018, 65, e12486. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.D.; Middleton, B.A.; Gous, R.M. Exogenous melatonin modifies rate of sexual maturation in domestic pullets. Poult. Sci. 2006, 85, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Marchena, A.M.; Holguin-Arevalo, M.S.; Martin-Partido, G.; Rodriguez, A.B.; Paredes, S.D.; Pariente, J.A. Anti-inflammatory effects of melatonin in a rat model of caerulein-induced acute pancreatitis. Cell Biochem. Funct. 2013, 31, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, J.; Szklarczyk, J.; Jaworek, A.K.; Nawrot-Porabka, K.; Leja-Szpak, A.; Bonior, J.; Kot, M. Protective effect of melatonin on acute pancreatitis. Int. J. Inflam. 2012, 2012, 173675. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Medzhitov, R. On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 2006, 7, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, E.; Kotsias, F.; Visentin, G.; Bruhns, P.; Savina, A.; Amigorena, S. Autonomous phagosomal degradation and antigen presentation in dendritic cells. Proc. Natl. Acad. Sci. USA 2012, 109, 14556–14561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burman, C.; Ktistakis, N.T. Autophagosome formation in mammalian cells. Semin. Immunopathol. 2010, 32, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Hoenerhoff, M.J.; Shockley, K.R.; Peddada, S.D.; Gerrish, K.E.; Sutton, D.; Cummings, C.A.; Wang, Y.; Julie, F.F.; Behl, M.; et al. Reduced Disc Shedding and Phagocytosis of Photoreceptor Outer Segment Contributes to Kava Kava Extract–induced Retinal Degeneration in F344/N Rats. Toxicol. Pathol. 2018, 46, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.C.; Tai, Y.I.; Huang, H.J.; Lin, A.M. Melatonin ameliorates arsenite-induced neurotoxicity: Involvement of autophagy and mitochondria. Mol. Neurobiol. 2015, 52, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Hu, S.; Shi, C.; Zhu, P.; Ma, Q.; Jin, Q.; Cao, F.; Tian, F.; Chen, Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC 1-HK 2-mPTP-mitophagy axis. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Guseo, A.; Klein, R. In vitro study on the immunological effect of bromelain and trypsin on mononuclear cells from humans. Eur. J. Med. Res. 2005, 10, 325–331. [Google Scholar] [PubMed]
- La Favor, J.D.; Dubis, G.S.; Yan, H.M.; White, J.D.; Nelson, M.A.M.; Anderson, E.J.; Hickner, R.C. Microvascular endothelial dysfunction in sedentary, obese humans is mediated by NADPH oxidase: influence of exercise training. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Hirai, H.; Yokota, A.; Sato, A.; Shoji, T.; Kashiwagi, T.; Iwasa, M.; Fujishiro, A.; Miura, Y.; Maekawa, T. Accelerated apoptosis of peripheral blood monocytes in Cebpb-deficient mice. Biochem. Biophys. Res. Commun. 2015, 464, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, L.E.; Li, W.; Cressman, D.E.; Peng, Y.; Ciliberto, G.; Poli, V.; Taub, R. CCAAT enhancer-binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J. Clin. Invest. 1998, 102, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.W.; Johnson, P.F.; Hennighausen, L.; Sterneck, E. The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998, 12, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.S.; Waage, J.; Rapin, N.; Bisgaard, H.C.; Larsen, F.S.; Porse, B.T. Temporal mapping of CEBPA and CEBPB binding during liver regeneration reveals dynamic occupancy and specific regulatory codes for homeostatic and cell cycle gene batteries. Genome Res. 2013, 23, 592–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cain, D.W.; O′Koren, E.G.; Kan, M.J.; Womble, M.; Sempowski, G.D.; Hopper, K.; Gunn, M.D.; Kelsoe, G. Identification of a tissue-specific, C/EBPβ-dependent pathway of differentiation for murine peritoneal macrophages. J. Immunol. 2013, 191, 4665–4675. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Y.D.; Zhou, J.; Yao, D.M.; Li, X. Identification of target genes of transcription factor CEBPB in acute promyelocytic leukemia cells induced by all-trans retinoic acid. Asian Pac. J. Trop. Med. 2013, 6, 473–480. [Google Scholar] [CrossRef]
- Marstrand, T.T.; Borup, R.; Willer, A.; Borregaard, N.; Sandelin, A.; Porse, B.T.; Theilgaard-Monch, K. A conceptual framework for the identification of candidate drugs and drug targets in acute promyelocytic leukemia. Leukemia 2010, 24, 1265–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochepied, T.; Berger, F.G.; Baumann, H.; Libert, C. α1-Acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003, 14, 25–34. [Google Scholar] [CrossRef]
- Shu, S.; Xu, Y.; Xie, L.; Ouyang, Y. The role of C/EBPβ phosphorylation in modulating membrane phospholipids repairing in LPS-induced human lung/bronchial epithelial cells. Gene 2017, 629, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Foka, P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem. J. 2002, 365, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Schrem, H.; Klempnauer, J.; Borlak, J. Liver-enriched transcription factors in liver function and development. Part II: The C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol. Rev. 2004, 56, 291–330. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yang, M.; Wu, H.; Ma, T.; He, C.; Chai, M.; Zhang, X.; Zhang, J.; Ding, F.; Wang, S.; et al. Effects of Aanat overexpression on the inflammatory responses and autophagy activity in the cellular and transgenic animal levels. Autophagy 2018, 1–20. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Tao, J.; Wu, H.; Zhang, L.; Yao, Y.; Liu, L.; Zhu, T.; Fan, H.; Cui, X.; Dou, H.; et al. Responses of Transgenic Melatonin-Enriched Goats on LPS Stimulation and the Proteogenomic Profiles of Their PBMCs. Int. J. Mol. Sci. 2018, 19, 2406. https://doi.org/10.3390/ijms19082406
Yang M, Tao J, Wu H, Zhang L, Yao Y, Liu L, Zhu T, Fan H, Cui X, Dou H, et al. Responses of Transgenic Melatonin-Enriched Goats on LPS Stimulation and the Proteogenomic Profiles of Their PBMCs. International Journal of Molecular Sciences. 2018; 19(8):2406. https://doi.org/10.3390/ijms19082406
Chicago/Turabian StyleYang, Minghui, Jingli Tao, Hao Wu, Lu Zhang, Yujun Yao, Lixi Liu, Tianqi Zhu, Hao Fan, Xudai Cui, Haoran Dou, and et al. 2018. "Responses of Transgenic Melatonin-Enriched Goats on LPS Stimulation and the Proteogenomic Profiles of Their PBMCs" International Journal of Molecular Sciences 19, no. 8: 2406. https://doi.org/10.3390/ijms19082406




