Two New Polyphenol Oxidase Genes of Tea Plant (Camellia sinensis) Respond Differentially to the Regurgitant of Tea Geometrid, Ectropis obliqua
Abstract
:1. Introduction
2. Results
2.1. cDNA Cloning and Sequence Analysis
2.2. The Phylogenetic Analysis
2.3. Expression of the Recombinant Protein in E. coli
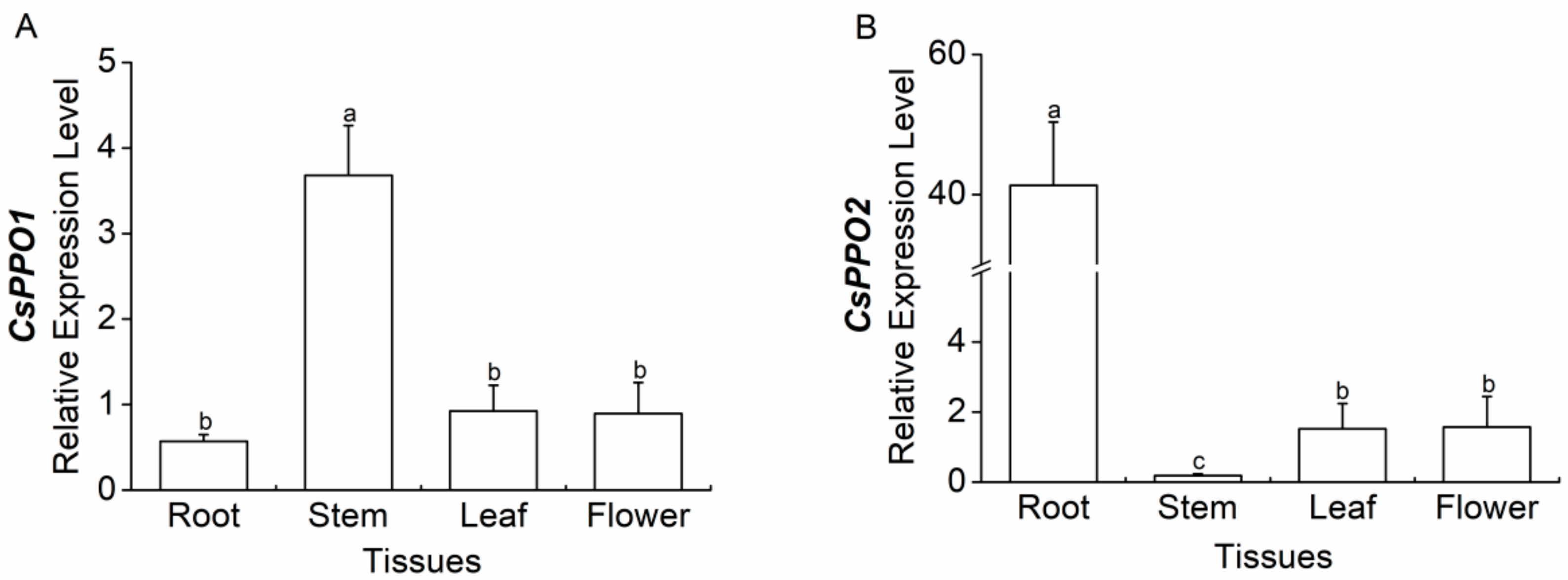
2.4. Expression of CsPPO1 and CsPPO2 in Different Tissues
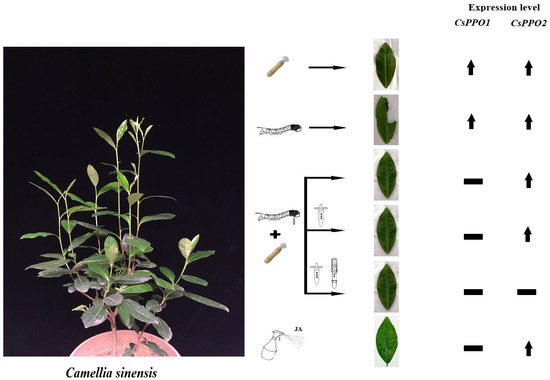
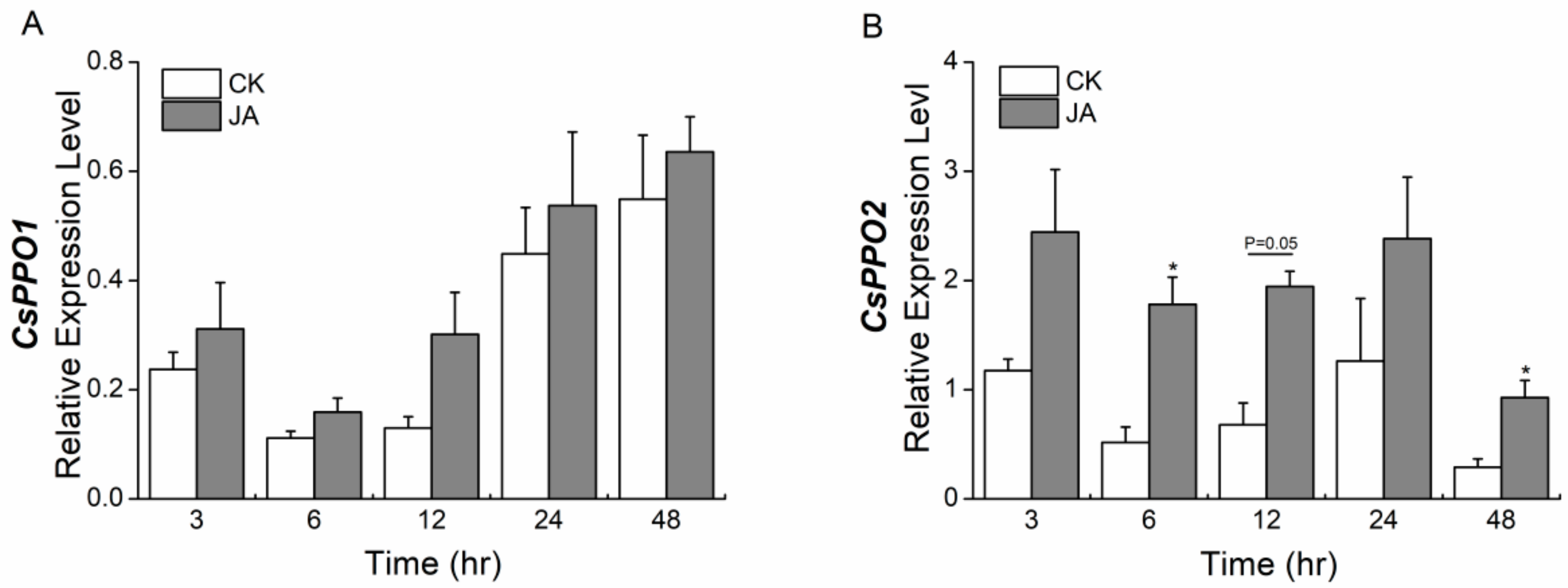
2.5. Jasmonic Acid Elicits the Expression of CsPPO1 and CsPPO2 Differentially
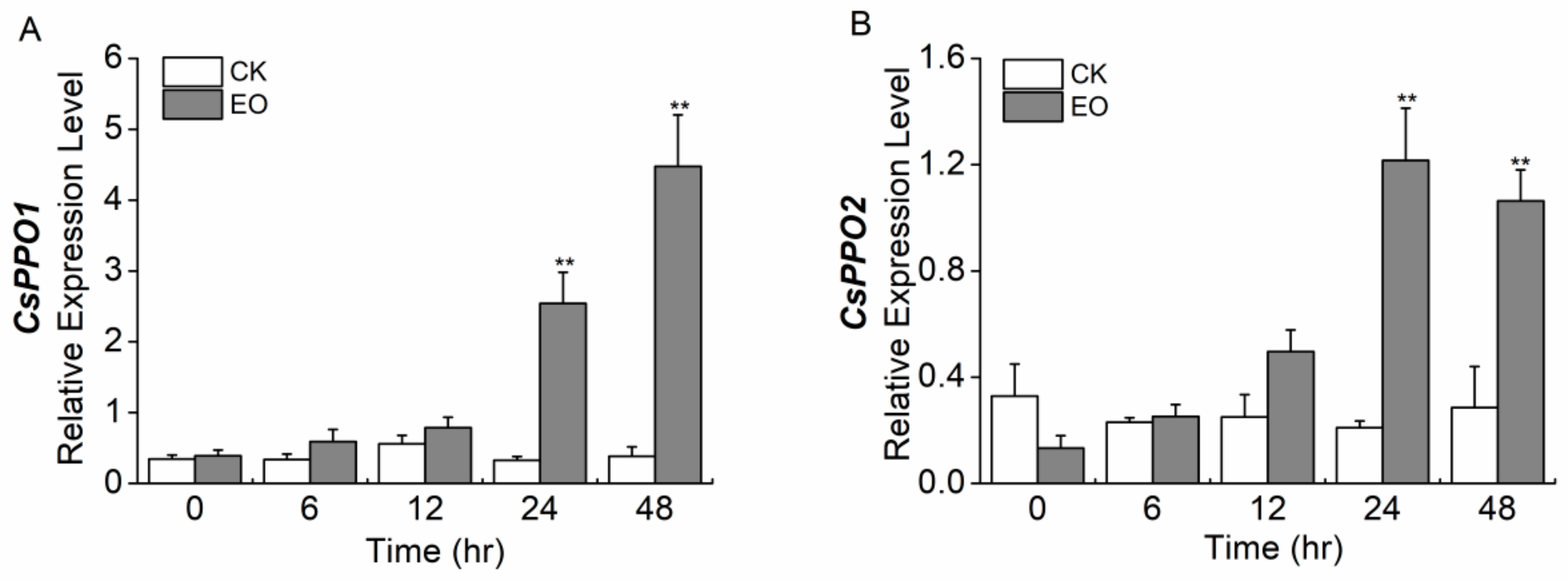
2.6. Infestation of Caterpillars Elicit the Expression of CsPPO1 and CsPPO2
2.7. Regurgitant Elicits the Expression of CsPPO1 and CsPPO2 Differentially
2.8. Separated Compounds of Regurgitant Elicit the Expression of CsPPO1 and CsPPO2 Differentially
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Regurgitant Collection and Separation
4.3. Tea Plants and Treatments
4.3.1. Different Tissues
4.3.2. JA Treatment
4.3.3. Caterpillar Infestation
4.3.4. Regurgitant Induction
4.3.5. Separated Regurgitant Induction
4.4. RNA Extraction and cDNA Synthesis
4.5. Cloning Full-Length CsPPO Gene Sequences
4.6. Bioinformatic and Phylogenetic Analysis
4.7. Recombinant Protein Expression in Escherichia coli
4.8. Real-Time PCR (qPCR) Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PPOs | Polyphenol oxidases |
| ORF | Open reading frame |
| JA | Jasmonic acid |
| MeJA | Methyl jasmonate |
| SA | Salicylic acid |
| ET | Ethylene |
| OS | Oral secretions |
| HAMPs | Herbivore-associated molecular patterns |
| CPB | Colorado potato beetles |
| CAAS | Chinese Academy of Agricultural Sciences |
| Mw | Molecular weight |
| pI | Isoelectric point |
| IPTG | Isopropyl-β-d-thiogalactopyranoside |
| SDS–PAGE | Sodium dodecylsulfate-polyacrylamide gel electrophoresis |
References
- Joy, R.W., IV; Sugiyama, M.; Fukuda, H.; Komamine, A. Cloning and characterization of polyphenol oxidase cDNAs of Phytolacca americana. Plant Physiol. 1995, 107, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yonekura-Sakakibara, K.; Sato, T.; Kikuchi, S.; Fukui, Y.; Fukuchi-Mizutani, M.; Ueda, T.; Nakao, M.; Tanaka, Y.; Kusumi, T. Aureusidin synthase: A polyphenol oxidase homolog responsible for flower coloration. Science 2000, 290, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Brunow, G.; Harris, P.J.; Dixon, R.A.; Schatz, P.F.; Boerjan, W. Lignification: Are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication. Recent Adv. Polyphen. Res. 2008, 1, 36–66. [Google Scholar]
- Yang, Z.-W.; Duan, X.-N.; Jin, S.; Li, X.-W.; Chen, Z.-M.; Ren, B.-Z.; Sun, X.-L. Regurgitant derived from the tea geometrid Ectropis obliqua suppresses wound-induced polyphenol oxidases activity in tea plants. J. Chem. Ecol. 2013, 39, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Du, M.-H.; Yan, Z.-W.; Hao, Y.-J.; Yan, Z.-T.; Si, F.-L.; Chen, B.; Qiao, L. Suppression of Laccase 2 severely impairs cuticle tanning and pathogen resistance during the pupal metamorphosis of Anopheles sinensis (Diptera: Culicidae). Parasites Vectors 2017, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Harel, E. Phenoloxidases and their significance in fruit and vegetables. Food Enzym. 1991, 1, 373–398. [Google Scholar]
- Si, H.; Zhou, Z.; Wang, X.; Ma, C. A novel molecular marker for the polyphenol oxidase gene located on chromosome 2B in common wheat. Mol. Breed. 2012, 30, 1371–1378. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Bhatt, R. Eggplant (Solanum melongena L.) polyphenol oxidase multi-gene family: A phylogenetic evaluation. Biotech 2015, 5, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, B.; Xiao, Q.; Li, H.; Sun, J. Cloning and expression analysis of litchi (Litchi chinensis sonn.) polyphenol oxidase gene and relationship with postharvest pericarp browning. PLoS ONE 2014, 9, e93982. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.; Donato, K.; Broadway, R.; Duffey, S. Impact of oxidized plant phenolics on the nutritional quality of dietar protein to a noctuid herbivore, Spodoptera exigua. J. Insect Physiol. 1992, 38, 277–285. [Google Scholar] [CrossRef]
- Thipyapong, P.; Hunt, M.D.; Steffens, J.C. Antisense downregulation of polyphenol oxidase results in enhanced disease susceptibility. Planta 2004, 220, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Bhonwong, A.; Stout, M.J.; Attajarusit, J.; Tantasawat, P. Defensive role of tomato polyphenol oxidases against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). J. Chem. Ecol. 2009, 35, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Berger, S.; Schaller, A.; Stintzi, A. Jasmonate-dependent induction of polyphenol oxidase activity in tomato foliage is important for defense against Spodoptera exigua but not against Manduca Sexta. BMC Plant Biol. 2014, 14, 257. [Google Scholar] [CrossRef] [PubMed]
- Redondo, D.; Venturini, M.E.; Oria, R.; Arias, E. Inhibitory effect of microwaved thinned nectarine extracts on polyphenol oxidase activity. Food Chem. 2016, 197, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, W.; Xiong, Z.; Zou, L.; Chen, J.; Liu, J.; Zhong, J. Different modes of inhibition for organic acids on polyphenol oxidase. Food Chem. 2016, 199, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.; Donato, K.; Del Vecchio, R.; Duffey, S. Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J. Chem. Ecol. 1989, 15, 2667–2694. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Mahanil, S.; Attajarusit, J.; Stout, M.J.; Thipyapong, P. Overexpression of tomato polyphenol oxidase increases resistance to common cutworm. Plant Sci. 2008, 174, 456–466. [Google Scholar] [CrossRef]
- Haruta, M.; Pedersen, J.A.; Constabel, C.P. Polyphenol oxidase and herbivore defense in trembling aspen (Populus tremuloides): cDNA cloning, expression, and potential substrates. Physiol. Plant. 2001, 112, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.J.; Cookson, A.; Allison, G.; Sullivan, M.L.; Winters, A.L. Gene expression patterns, localization, and substrates of polyphenol oxidase in red clover (Trifolium pratense L.). J. Agric. Food Chem. 2013, 61, 7421–7430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Z.; Qiao-Ying, M.A.; Zhang, S.; Lv, L.M.; Luo, J.Y.; Wang, C.Y.; Cui, J.J. Cloning of a polyphenol oxidase gene (GhPPO1) of Gossypium hirsutum and its role in cotton after Helicoverpa armigera feeding. Sci. Agric. Sin. 2014, 47, 3174–3183. [Google Scholar]
- Li, C.; Li, D.; Li, J.; Shao, F.; Lu, S. Characterization of the polyphenol oxidase gene family reveals a novel microRNA involved in posttranscriptional regulation of PPOs in Salvia miltiorrhiza. Sci. Rep. 2017, 7, 44622. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, Q.; Lu, H.; Wu, C.; Lu, F.; Tang, J. Increased activities of peroxidase and polyphenol oxidase enhance cassava resistance to Tetranychus urticae. Exp. Appl. Acarol. 2017, 71, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Thipyapong, P.; Stout, M.J.; Attajarusit, J. Functional analysis of polyphenol oxidases by antisense/sense technology. Molecules 2007, 12, 1569–1595. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.; Heywood, S.; Farrar, K.; Donnison, I.; Thomas, A.; Webb, K.J. Identification of an extensive gene cluster among a family of PPOs in Trifolium pratense L. (red clover) using a large insert BAC library. BMC Plant Biol. 2009, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, L.T.; Taylor, J.S.; Constabel, C.P. The polyphenol oxidase gene family in land plants: Lineage-specific duplication and expansion. BMC Genom. 2012, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.M.; Eannetta, N.T.; Yu, H.; Prince, J.P.; de Vicente, M.C.; Tanksley, S.D.; Steffens, J.C. Organisation of the tomato polyphenol oxidase gene family. Plant Mol. Biol. 1993, 21, 1035–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, Z.S.; Biao, X.I. Cloning and alignment of polyphenol oxidase cDNA of tea plant. J. Tea Sci. 2001, 2, 94–98. [Google Scholar]
- Liu, S.; Han, B. Differential expression pattern of an acidic 9/13-lipoxygenase in flower opening and senescence and in leaf response to phloem feeders in the tea plant. BMC Plant Biol. 2010, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Cui, L.; Ge, J.; Wang, M.X.; Liu, L.F.; Yu, X.P.; Zhang, Q.H.; Han, B.Y. Behavioral responses for evaluating the attractiveness of specific tea shoot volatiles to the tea green leafhopper, Empoaca vitis. Insect Sci. 2012, 19, 229–238. [Google Scholar] [CrossRef]
- Chen, Y. Biological control progress of Ectropis obliqua prout. Nat. Enemies Insects 2001, 23, 181–184. [Google Scholar]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chung, S.H.; Peiffer, M.; Rosa, C.; Hoover, K.; Zeng, R.; Felton, G.W. Herbivore oral secreted bacteria trigger distinct defense responses in preferred and non-preferred host plants. J. Chem. Ecol. 2016, 42, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Halitschke, R.; Kessler, A.; Schittko, U. Merging molecular and ecological approaches in plant-insect interactions. Curr. Opin. Plant Biol. 2001, 4, 351–358. [Google Scholar] [CrossRef]
- Musser, R.O.; Farmer, E.; Peiffer, M.; Williams, S.A.; Felton, G.W. Ablation of caterpillar labial salivary glands: Technique for determining the role of saliva in insect–plant interactions. J. Chem. Ecol. 2006, 32, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Musser, R.O.; Hum-Musser, S.M.; Lee, H.K.; DesRochers, B.L.; Williams, S.A.; Vogel, H. Caterpillar labial saliva alters tomato plant gene expression. J. Chem. Ecol. 2012, 38, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Dowd, P.F.; Johnson, E.T.; Hum-Musser, S.M.; Musser, R.O. Effects of elevated peroxidase levels and corn earworm feeding on gene expression in tomato. J. Chem. Ecol. 2012, 38, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.N. Change of polyphenol oxidase activity during oolong tea process. J. Food Nutr. Sci. 2015, 3, 88–93. [Google Scholar] [CrossRef]
- Teng, J.; Gong, Z.; Deng, Y.; Chen, L.; Li, Q.; Shao, Y.; Lin, L.; Xiao, W. Purification, characterization and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis). LWT Food Sci. Technol. 2017, 84, 263–270. [Google Scholar] [CrossRef]
- Halder, J.; Tamuli, P.; Bhaduri, A.N. Isolation and characterization of polyphenol oxidase from Indian tea leaf (Camellia sinensis). J. Nutr. Biochem. 1998, 9, 75–80. [Google Scholar] [CrossRef]
- Wahler, D.; Gronover, C.S.; Richter, C.; Foucu, F.; Twyman, R.M.; Moerschbacher, B.M.; Fischer, R.; Muth, J.; Prüfer, D. Polyphenol oxidase silencing affects latex coagulation in taraxacum species. Plant Physiol. 2009, 151, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Mita, G.; Durante, M.; Arlorio, M.; De Paolis, A. Isolation of a polyphenol oxidase (PPO) cDNA from artichoke and expression analysis in wounded artichoke heads. Plant Physiol. Biochem. 2013, 68, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Deng, Z.; Liu, C.; Zhao, M.; Guo, H.; Xia, Z.; Liu, H. Molecular cloning, expression profiles, and characterization of a novel polyphenol oxidase (PPO) gene in Hevea brasiliensis. Biosci. Biotechnol. Biochem. 2014, 78, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhao, P.; Wang, B.; Tariq, P.; Zhao, F.; Zhao, M.; Wang, Q.; Yang, T.; Fang, J. Overexpression of polyphenol oxidase gene in strawberry fruit delays the fungus infection process. Plant Mol. Boil. Report. 2016, 34, 592–606. [Google Scholar] [CrossRef]
- Nakayama, T.; Sato, T.; Fukui, Y.; Yonekura-Sakakibara, K.; Hayashi, H.; Tanaka, Y.; Kusumi, T.; Nishino, T. Specificity analysis and mechanism of aurone synthesis catalyzed by aureusidin synthase, a polyphenol oxidase homolog responsible for flower coloration. FEBS Lett. 2001, 499, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Shetty, S.M.; Chandrashekar, A.; Venkatesh, Y.P. Eggplant polyphenol oxidase multigene family: Cloning, phylogeny, expression analyses and immunolocalization in response to wounding. Phytochemistry 2011, 72, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Constabel, C.P. The polyphenol oxidase gene family in poplar: Phylogeny, differential expression and identification of a novel, vacuolar isoform. Planta 2011, 234, 799. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Moinuddin, S.G.; Helms, G.L.; Hishiyama, S.; Eichinger, D.; Davin, L.B.; Lewis, N.G. (+)-Larreatricin hydroxylase, an enantio-specific polyphenol oxidase from the creosote bush (Larrea tridentata). Proc. Natl. Acad. Sci. USA 2003, 100, 10641–10646. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.D.; Voelckel, C.; Hartl, M.; Schmidt, S.; Baldwin, I.T. Specificity in ecological interactions. Attack from the same lepidopteran herbivore results in species-specific transcriptional responses in two solanaceous host plants. Plant Physiol. 2005, 138, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Pan, L.P.; Yu, S.L.; Li, H.H. Cloning, microbial expression and structure-activity relationship of polyphenol oxidases from Camellia sinensis. J. Biotechnol. 2010, 145, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Constabel, C.P. Three polyphenol oxidases from hybrid poplar are differentially expressed during development and after wounding and elicitor treatment. Physiol. Plant. 2004, 122, 344–353. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, J.; Chen, M.L.; Ou Yang, Y.; Luo, X.Q. Research on the anti-inflammatory and analgesic actions of tea plant root. Res. Pract. Chin. Med. 2012, 4, 39–41. [Google Scholar]
- Castañera, P.; Steffens, J.; Tingey, W. Biological performance of colorado potato beetle larvae on potato genotypes with differing levels of polyphenol oxidase. J. Chem. Ecol. 1996, 22, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Constabel, C.P.; Bergey, D.R.; Ryan, C.A. Polyphenol oxidase as a component of the inducible defense response in tomato against herbivores. Recent Adv. Phytochem. 1996, 30, 231–252. [Google Scholar]
- He, J.; Chen, F.; Chen, S.; Lv, G.; Deng, Y.; Fang, W.; Liu, Z.; Guan, Z.; He, C. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Physiol. 2011, 168, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Ralph, S.G.; Mellway, R.; White, R.; Heath, M.C.; Bohlmann, J.; Constabel, C.P. The transcriptional response of hybrid poplar (Populus trichocarpa × P. deltoids to infection by melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol. Plant Microbe Interact. 2007, 20, 816–831. [Google Scholar]
- Boss, P.K.; Gardner, R.C.; Janssen, B.-J.; Ross, G.S. An apple polyphenol oxidase cdna is up-regulated in wounded tissues. Plant Mol. Biol. 1995, 27, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Sawyer, B.J.; Bucheli, C.S.; Robinson, S.P. Polyphenol oxidase is induced by chilling and wounding in pineapple. Funct. Plant Biol. 2001, 28, 181–191. [Google Scholar] [CrossRef]
- Thipyapong, P.; Steffens, J.C. Differential expression and turnover of the tomato polyphenol oxidase gene family during vegetative and reproductive development. Plant Physiol. 1997, 113, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Tang, L.; Hou, Y.; Wang, P.; Yang, H.; Wei, C.L. Differential transcriptome analysis of leaves of tea plant (Camellia sinensis) provides comprehensive insights into the defense responses to Ectropis obliqua attack using RNA-seq. Funct. Integr. Genom. 2016, 16, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Musser, R.O.; Hummusser, S.M.; Eichenseer, H.; Peiffer, M.; Ervin, G.; Murphy, J.B.; Felton, G.W. Herbivory: Caterpillar saliva beats plant defences. Nature 2002, 416, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Glauser, G.; Robert, C.A. Induced immunity against belowground insect herbivores-activation of defenses in the absence of a jasmonate burst. J. Chem. Ecol. 2012, 38, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Gaffor, I.; Acevedo, F.E.; Helms, A.; Chuang, W.P.; Tooker, J.; Felton, G.W.; Luthe, D.S. Maize plants recognize herbivore-associated cues from caterpillar frass. J. Chem. Ecol. 2015, 41, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Baldwin, I.T. Nitrogen supply influences herbivore-induced direct and indirect defenses and transcriptional responses in Nicotiana attenuata. Plant Physiol. 2004, 135, 496–506. [Google Scholar] [CrossRef] [PubMed]





| Name | CsPPO1 | CsPPO2 | CsPPO |
|---|---|---|---|
| CsPPO1 | 100.0 | 68.45 | 69.83 |
| CsPPO2 | 71.68 | 100.0 | 71.14 |
| CsPPO | 73.27 | 75.27 | 100.0 |
| Primers | Purpose | Primer Sequence (5′-3′) |
|---|---|---|
| GSP1-3 | 3′-RACE | AATGTGGATCGGATGTGG |
| NGSP1-3 | 3′-RACE | CAGAGACCGAAGAAATCAAG |
| GSP2-3 | 3′-RACE | GGTTTGTGTTCTATGATGAG |
| NGSP2-3 | 3′-RACE | AGAAGGATGATGAAGAGGAG |
| GSP1-5 | 5′-RACE | GAGCCATGAGTTGTGGACTTGAAG |
| NGSP1-5 | 5′-RACE | TGGTGATAAGCTCCGTCGCAATAA |
| GSP2-5 | 5′-RACE | ATCCGACCCGTTGTAATCCA |
| NGSP2-5 | 5′-RACE | CGAAGAAGTGGAGGTAGTAT |
| UPM | RACE | Long primer: CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT |
| Short primer: CTAATACGACTCACTATAGGGC | ||
| PPO1-full1 | Clone | ATGAATTCTCTTCCACCATCA |
| PPO1-full2 | Clone | TTAGGAATCAAACTCAATCTTG |
| PPO2-full1 | Clone | ATGGCTTCTTTTCCACCTTC |
| PPO2-full2 | Clone | TCAAGAATCAAACTCTATCTTGA |
| PPO1-PF | protein | CTGGTTCCGCGTGGATCCATGAATTCTCTTCCACCATCATGCA |
| PPO1-PR | protein | CGCTCGAGTCGACCCGGGTTAGGAATCAAACTCAATCTTG |
| PPO2-PF | protein | CTGGTTCCGCGTGGATCCATGGCTTCTTTTCCACCTTC |
| PPO2-PR | protein | CGCTCGAGTCGACCCGGGTCAAGAATCAAACTCTATCTTGA |
| GAPDH-F | QPCR | GACTGGAGAGGTGGAAGAGC |
| GAPDH-R | QPCR | AGCCATTCCAGTCAATTTCC |
| PPO1-RTF | QPCR | CCATCTGGAAGAGTTTGGGT |
| PPO1-RTR | QPCR | CCTTCACTTTGACAGGCTGA |
| PPO2-RTF | QPCR | CGGAATGCCAATGCCTGCAA |
| PPO2-RTR | QPCR | AGTTGGATCCGACCCGTTGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhang, J.; Zhang, X.; Yu, Y.; Bian, W.; Zeng, Z.; Sun, X.; Li, X. Two New Polyphenol Oxidase Genes of Tea Plant (Camellia sinensis) Respond Differentially to the Regurgitant of Tea Geometrid, Ectropis obliqua. Int. J. Mol. Sci. 2018, 19, 2414. https://doi.org/10.3390/ijms19082414
Huang C, Zhang J, Zhang X, Yu Y, Bian W, Zeng Z, Sun X, Li X. Two New Polyphenol Oxidase Genes of Tea Plant (Camellia sinensis) Respond Differentially to the Regurgitant of Tea Geometrid, Ectropis obliqua. International Journal of Molecular Sciences. 2018; 19(8):2414. https://doi.org/10.3390/ijms19082414
Chicago/Turabian StyleHuang, Chen, Jin Zhang, Xin Zhang, Yongchen Yu, Wenbo Bian, Zhongping Zeng, Xiaoling Sun, and Xinghui Li. 2018. "Two New Polyphenol Oxidase Genes of Tea Plant (Camellia sinensis) Respond Differentially to the Regurgitant of Tea Geometrid, Ectropis obliqua" International Journal of Molecular Sciences 19, no. 8: 2414. https://doi.org/10.3390/ijms19082414
APA StyleHuang, C., Zhang, J., Zhang, X., Yu, Y., Bian, W., Zeng, Z., Sun, X., & Li, X. (2018). Two New Polyphenol Oxidase Genes of Tea Plant (Camellia sinensis) Respond Differentially to the Regurgitant of Tea Geometrid, Ectropis obliqua. International Journal of Molecular Sciences, 19(8), 2414. https://doi.org/10.3390/ijms19082414