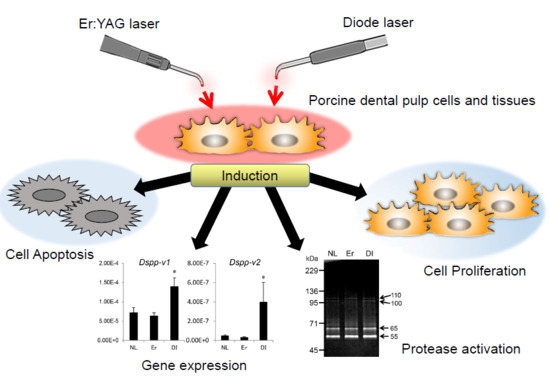
Effects of Er:YAG and Diode Laser Irradiation on Dental Pulp Cells and Tissues
Abstract
:1. Introduction
2. Results
2.1. Cell Proliferation Rate of PPU-7
2.2. Apoptosis of PPU-7
2.3. Effect of LI on Differentiation and Gene Expression in PPU-7
2.4. Effect of LI on Mineralization Induction in PPU-7
2.5. Effect of LI on Protease Activation in Dental Pulp Tissue
2.6. Effect of LI on TGF-β Activation
3. Discussion
4. Methods
4.1. LI of Porcine Dental Pulp Cells (PPU-7)
4.2. Cell Proliferation Assay
4.3. Assessment of Apoptosis by Immunohistochemistry
4.4. Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.5. ALP Assay
4.6. Formation of Precipitated Nodules in PPU-7 Cells
4.7. LI of Porcine Dental Pulp Tissues and Protein Extraction
4.8. Zymography
4.9. In Vitro Activation of Latent TGF-β1 by LI
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MTS | 3-[4,5,dimethylthiazol-2-yl]-5-[3-carboxymethoxy-phenyl]-2-[4-sulfophenyl]-2H-tetrazolium inner |
| mRNA | messenger ribonucleic acid |
| qPCR | quantitative polymerase chain reaction |
| EDTA | ethylenediaminetetraacetic acid |
| SDS | sodium dodecyl sulfate |
| PAGE | polyacrylamide gel electrophoresis |
| kDa | kilodalton |
| HeNe | helium-neon |
| HPFs | high-power fields |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
Appendix A
Appendix A.1. Immortalization of Porcine Pulp Cells and ALP Expression


Appendix A.2. Cell Proliferation Rate
Appendix A.3. Gene Expression in PPU-7 and PPU-16 Cell Lines

Appendix A.4. Cell Line Isolation, Transfection, and Establishment from Porcine Dental Pulp Cells
Appendix A.5. ALP-HPDL System




| Gene | Sequence (5′→3′) | Size (bp) | qPCR Protocol (45 Cycles) | |||
|---|---|---|---|---|---|---|
| Mmp2 | F | CCGACGTGGCCAATTACAAC | 96 | |||
| R | GGTCCAGATCAGGCGTGTAG | |||||
| Mmp20 | F | ATGCAGCTTACGAAGTGGCT | 95 | |||
| R | GGGAGGACCTTGCATTTGGA | |||||
| Dspp-v1 | F | CCCAGAAACCCAATCAGAGA | 300 | |||
| R | TATGTGTTTTGCTGGGTCCA | |||||
| Dspp-v2 | F | CCCAGAAACCCAATCAGAGA | 149 | |||
| R | GGGAAGGAAGGGGAGAATTT | |||||
| Runx2 | F | CAACTTCCTGTGCTCTGTGC | 119 | Denaturation | 95 °C | 10 s |
| R | CCGCCATGACAGTAACCACA | |||||
| OC | F | CCAGGCAGATGCAAAGCCTA | 96 | Annealing | 60 °C | 10 s |
| R | CGCCTGAGTCTCTTCACCAC | |||||
| Col II | F | CCAGATTGAGAGCATCCGCA | 142 | Extension | 72 °C | 10 s |
| R | CATGGCGTCCAAAGTGCATC | |||||
| Tgf-β1 | F | GCCTGCTGAGGCTCAAGTTA | 131 | |||
| R | ATCAAAGGACAGCCACTCCG | |||||
| Tgf-β2 | F | GCGCTACATCGACAGCAAAG | 143 | |||
| R | TGCAGCAGGGACAGTGTAAG | |||||
| Tgf-β3 | F | CTGTGCGTGAATGGCTCTTG | 93 | |||
| R | TATCCCCGTTGGGCTGAAAG | |||||
| Gapdh | F | CCATCACCATCTTCCAGGAG | 346 | |||
| R | ACAGTCTTCTGGGTGGCAGT | |||||
References
- Yilmaz, H.G.; Albaba, M.R.; Caygur, A.; Cengiz, E.; Boke-Karacaoglu, F.; Tumer, H. Treatment of recurrent aphthous stomatitis with Er,Cr:YSGG laser irradiation: A randomized controlled split mouth clinical study. J. Photochem. Photobiol. B 2017, 170, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Ebadifar, A.; Kabiri, S.; Badiee, M.R.; Abdolazimi, Z.; Amdjadi, P. Comparative effectiveness of low level laser therapy and transcutaneous electric nerve stimulation on temporomandibular joint disorders. J. Lasers Med. Sci. 2017, 8, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Jafri, Z.; Sultan, N.; Sawai, M.; Daing, A. Periodontal flap surgery along with vestibular deepening with diode laser to increase attached gingiva in lower anterior teeth: A prospective clinical study. J. Nat. Sci. Biol. Med. 2018, 9, 72–76. [Google Scholar] [PubMed]
- Zeini Jahromi, N.; Ghapanchi, J.; Pourshahidi, S.; Zahed, M.; Ebrahimi, H. Clinical evaluation of high and low-level laser treatment (CO2vsInGaAlp diode laser) for recurrent aphthous stomatitis. J. Dent. 2017, 18, 17–23. [Google Scholar]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-microm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Ando, Y.; Watanabe, H.; Ishikawa, I. In vitro studies on laser scaling of subgingival calculus with an Erbium:YAG Laser. J. Periodontol. 1994, 65, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Miura, M.; Akiyama, F.; Nakagawa, N.; Tanaka, J.; Oda, S.; Watanabe, H.; Ishikawa, I. In vitro evaluation of Er:YAG laser scaling of subgingival calculus in comparison with ultrasonic scaling. J. Periodontal Res. 2000, 35, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.M.; Aoki, A.; Ichinose, S.; Ishikawa, I. Morphological analysis of cementum and root dentin after Er:YAG laser irradiation. Lasers Surg. Med. 2002, 31, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.M.; Aoki, A.; Ichinose, S.; Ishikawa, I. Ultrastructural analysis of bone tissue irradiated by Er:YAG laser. Lasers Surg. Med. 2002, 31, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.M.; Aoki, A.; Ichinose, S.; Yoshino, T.; Yamada, S.; Ishikawa, I. Scanning electron microscopy and fourier transformed infrared spectroscopy analysis of bone removal using Er:YAG and CO2 lasers. J. Periodontol. 2002, 73, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Sawabe, M.; Aoki, A.; Komaki, M.; Iwasaki, K.; Ogita, M.; Izumi, Y. Gingival tissue healing following Er:YAG Laser ablation compared to electrosurgery in rats. Lasers Med. Sci. 2015, 30, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Zare, D.; Haerian, A.; Molla, R.; Vaziri, F. Evaluation of the effects of diode (980 nm) laser on gingival inflammation after nonsurgical periodontal therapy. J. Lasers Med. Sci. 2014, 5, 27–31. [Google Scholar] [PubMed]
- Ortega-Concepcion, D.; Cano-Duran, J.A.; Pena-Cardelles, J.F.; Paredes-Rodriguez, V.M.; Gonzalez-Serrano, J.; Lopez-Quiles, J. The application of diode laser in the treatment of oral soft tissues lesions. A literature review. J. Clin. Exp. Dent. 2017, 9, e925–e928. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. Effect of 808 nm diode laser on swimming behavior, food vacuole formation and endogenous ATP production of paramecium primaurelia (Protozoa). Photochem. Photobiol. 2015, 91, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. The protozoan, paramecium primaurelia, as a non-sentient model to test laser light irradiation: The effects of an 808 nm infrared laser diode on cellular respiration. Altern. Lab. Anim. 2015, 43, 155–162. [Google Scholar] [PubMed]
- Amaroli, A.; Parker, S.; Dorigo, G.; Benedicenti, A.; Benedicenti, S. Paramecium: A promising non-animal bioassay to study the effect of 808 nm infrared diode laser photobiomodulation. Photomed. Laser Surg. 2015, 33, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-nm laser therapy with a flat-top handpiece photobiomodulates mitochondria activities of paramecium primaurelia (protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Oron, U.; Gedanken, A.; Lubart, R. Low-level visible light (llvl) irradiation promotes proliferation of mesenchymal stem cells. Lasers Med. Sci. 2013, 28, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Santinoni, C.D.; Oliveira, H.F.; Batista, V.E.; Lemos, C.A.; Verri, F.R. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: A systematic review. J. Photochem. Photobiol. B 2017, 169, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Imai, M.; Goto, Y.; Murakami, N. Pathological mineralization in a serially passaged cell line from rat pulp. J. Oral Pathol. Med. 1993, 22, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Yokota, M.; Ohishi, K.; Nishikawa, S.; Shinohara, H.; Wakano, Y.; Ishida, H. 1α,25-dihydroxyvitamin D3 stimulation of osteopontin expression in rat clonal dental pulp cells. Arch. Oral Biol. 1994, 39, 775–782. [Google Scholar] [CrossRef]
- MacDougall, M.; Selden, J.K.; Nydegger, J.R.; Carnes, D.L. Immortalized mouse odontoblast cell line MO6-G3 application for in vitro biocompatibility testing. Am. J. Dent. 1998, 11, S11–S16. [Google Scholar] [PubMed]
- Thonemann, B.; Schmalz, G. Bovine dental papilla-derived cells immortalized with HPV 18 E6/E7. Eur. J. Oral Sci. 2000, 108, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamakoshi, Y.; Simmer, J.P.; Ishikawa, I.; Hu, J.C. Establishment of porcine pulp-derived cell lines and expression of recombinant dentin sialoprotein and recombinant dentin matrix protein-1. Eur. J. Oral Sci. 2007, 115, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kamata, N.; Fujimoto, R.; Tomonari, M.; Taki, M.; Nagayama, M.; Yasumoto, S. Immortalization of human dental papilla, dental pulp, periodontal ligament cells and gingival fibroblasts by telomerase reverse transcriptase. J. Oral Pathol. Med. 2004, 33, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Schweikl, H.; Thonemann, B.; D’Souza, R.N.; Schmalz, G. Human pulp-derived cells immortalized with Simian Virus 40 T-antigen. Eur. J. Oral Sci. 2006, 114, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Begue-Kirn, C.; Smith, A.J.; Loriot, M.; Kupferle, C.; Ruch, J.V.; Lesot, H. Comparative analysis of TGF beta s, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int. J. Dev. Biol. 1994, 38, 405–420. [Google Scholar] [PubMed]
- Yang, X.; van der Kraan, P.M.; van den Dolder, J.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. STRO-1 selected rat dental pulp stem cells transfected with adenoviral-mediated human bone morphogenetic protein 2 gene show enhanced odontogenic differentiation. Tissue Eng. 2007, 13, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Tucker, A.; Sharpe, P.T. Organized tooth-specific cellular differentiation stimulated by BMP4. J. Dent. Res. 2005, 84, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Tjaderhane, L.; Koivumaki, S.; Paakkonen, V.; Ilvesaro, J.; Soini, Y.; Salo, T.; Metsikko, K.; Tuukkanen, J. Polarity of mature human odontoblasts. J. Dent. Res. 2013, 92, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Sun, X.; Bai, S.; Li, S.; Shi, J. Odontoblast-like cell differentiation and dentin formation induced with TGF-β1. Arch. Oral Biol. 2011, 56, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Yamakoshi, Y.; Yamazaki, H.; Karakida, T.; Chiba, R.; Hu, J.C.; Nagano, T.; Yamamoto, R.; Simmer, J.P.; Margolis, H.C.; et al. The dynamics of TGF-β in dental pulp, odontoblasts and dentin. Sci. Rep. 2018, 8, 4450. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, T.; Xing, D.; Wang, F.; Pei, Y.; Wei, X. Single cell analysis of PKC activation during proliferation and apoptosis induced by laser irradiation. J. Cell. Physiol. 2006, 206, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xing, D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J. Biomed. Sci. 2009, 16, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefer, G.; Oron, U.; Irintchev, A.; Wernig, A.; Halevy, O. Skeletal muscle cell activation by low-energy laser irradiation: A role for the MAPK/ERK pathway. J. Cell. Physiol. 2001, 187, 73–80. [Google Scholar] [CrossRef]
- Aleksic, V.; Aoki, A.; Iwasaki, K.; Takasaki, A.A.; Wang, C.Y.; Abiko, Y.; Ishikawa, I.; Izumi, Y. Low-level Er:YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med. Sci. 2010, 25, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Ogita, M.; Tsuchida, S.; Aoki, A.; Satoh, M.; Kado, S.; Sawabe, M.; Nanbara, H.; Kobayashi, H.; Takeuchi, Y.; Mizutani, K.; et al. Increased cell proliferation and differential protein expression induced by low-level Er:YAG laser irradiation in human gingival fibroblasts: Proteomic analysis. Lasers Med. Sci. 2015, 30, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Pourzarandian, A.; Watanabe, H.; Ruwanpura, S.M.; Aoki, A.; Ishikawa, I. Effect of low-level Er:YAG Laser irradiation on cultured human gingival fibroblasts. J. Periodontol. 2005, 76, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Niwa, T.; Yamakawa, S.; Saito, M.; Yamazaki, Y.; Hosoya, N.; Gomi, K.; Yamakoshi, Y. The effects of Er:YAG laser irradiation on cultured human periodontal ligament cells. J. Jpn. Soc. Laser Dent. 2016, 27, 84–89. (In Japanese) [Google Scholar] [CrossRef]
- Yoshida, M.; Kumamoto, H.; Ooya, K.; Mayanagi, H. Histopathological and immunohistochemical analysis of calcifying odontogenic cysts. J. Oral Pathol. Med. 2001, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Hirata, K.; Taki, E.; Shinoda, K.; Gomyo, S.; Sekine, I. The effect of GaAlAs laser power on cultured cells. J. Jpn. Soc. Laser Dent. 1995, 38, 1408–1423. (In Japanese) [Google Scholar]
- Gross, A.J.; Jelkmann, W. Helium-neon laser irradiation inhibits the growth of kidney epithelial cells in culture. Lasers Surg. Med. 1990, 10, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Lubart, R.; Wollman, Y.; Friedmann, H.; Rochkind, S.; Laulicht, I. Effects of visible and near-infrared lasers on cell cultures. J. Photochem. Photobiol. B 1992, 12, 305–310. [Google Scholar] [CrossRef]
- Breitbart, H.; Levinshal, T.; Cohen, N.; Friedmann, H.; Lubart, R. Changes in calcium transport in mammalian sperm mitochondria and plasma membrane irradiated at 633 nm (HeNe laser). J. Photochem. Photobiol. B 1996, 34, 117–121. [Google Scholar] [CrossRef]
- Pan, T.L.; Wang, P.W.; Lee, W.R.; Fang, C.L.; Chen, C.C.; Huang, C.M.; Fang, J.Y. Systematic evaluations of skin damage irradiated by an erbium:YAG laser: Histopathologic analysis, proteomic profiles, and cellular response. J. Dermatol. Sci. 2010, 58, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Barak, A.; Goldkorn, T.; Morse, L.S. Laser induces apoptosis and ceramide production in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Harrington, H.A.; Ho, K.L.; Ghosh, S.; Tung, K.C. Construction and analysis of a modular model of caspase activation in apoptosis. Theor. Biol. Med. Model. 2008, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, S.; Hamada, S.; Asou, H.; Kuida, K.; Uchiyama, Y.; Yoshida, H.; Miura, M. Caspase-9 activation revealed by semaphorin 7A cleavage is independent of apoptosis in the aged olfactory bulb. J. Neurosci. 2009, 29, 11385–11392. [Google Scholar] [CrossRef] [PubMed]
- Shigetani, Y.; Ohkura, N.; Yoshiba, K.; Ohshima, H.; Hosoya, A.; Yoshiba, N.; Okiji, T. GaAlAs laser-induced pulp mineralization involves dentin matrix protein 1 and osteopontin expression. Oral Dis. 2016, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Oida, S.; Yamakoshi, Y. Dentin sialophosphoprotein-derived proteins in the dental pulp. J. Dent. Res. 2015, 94, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Palosaari, H.; Ding, Y.; Larmas, M.; Sorsa, T.; Bartlett, J.D.; Salo, T.; Tjaderhane, L. Regulation and interactions of MT1-MMP and MMP-20 in human odontoblasts and pulp tissue in vitro. J. Dent. Res. 2002, 81, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Sulkala, M.; Tervahartiala, T.; Sorsa, T.; Larmas, M.; Salo, T.; Tjaderhane, L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch. Oral Biol. 2007, 52, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sulkala, M.; Larmas, M.; Sorsa, T.; Salo, T.; Tjaderhane, L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J. Dent. Res. 2002, 81, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Sulkala, M.; Paakkonen, V.; Larmas, M.; Salo, T.; Tjaderhane, L. Matrix metalloproteinase-13 (MMP-13, collagenase-3) is highly expressed in human tooth pulp. Connect. Tissue Res. 2004, 45, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Pashley, D.H.; Tay, F.R.; Gobbi, P.; Orsini, G.; Ruggeri, A., Jr.; Carrilho, M.; Tjaderhane, L.; Di Lenarda, R.; Breschi, L. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: Correlative FEI-SEM/TEM analysis. J. Biomed. Mater. Res. A 2009, 88, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Papa, V.; Nato, F.; Carrilho, M.; Tjaderhane, L.; Ruggeri, A., Jr.; Gobbi, P.; Mazzotti, G.; Tay, F.R.; Pashley, D.H.; et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J. Dent. 2011, 39, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloan, A.J.; Rutherford, R.B.; Smith, A.J. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch. Oral Biol. 2000, 45, 173–177. [Google Scholar] [CrossRef]
- Chen, S.; Gu, T.T.; Sreenath, T.; Kulkarni, A.B.; Karsenty, G.; MacDougall, M. Spatial expression of Cbfa1/Runx2 isoforms in teeth and characterization of binding sites in the DSPP gene. Connect. Tissue Res. 2002, 43, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red s stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.I.; Jussila, T.; Fusenig, N.E.; Stenback, F. TGF-β isoforms are differentially expressed in increasing malignant grades of HaCaT keratinocytes, suggesting separate roles in skin carcinogenesis. J. Pathol. 2000, 190, 579–588. [Google Scholar] [CrossRef]
- Sloan, A.J.; Smith, A.J. Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-β isoforms 1–3 in vitro. Arch. Oral Biol. 1999, 44, 149–156. [Google Scholar] [CrossRef]
- Nocera, M.; Chu, T.M. Characterization of latent transforming growth factor-beta from human seminal plasma. Am. J. Reprod. Immunol. 1995, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G.; Aznavoorian, S.; Liotta, L.A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 1993, 9, 541–573. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [PubMed]
- Nagano, T.; Oida, S.; Suzuki, S.; Iwata, T.; Yamakoshi, Y.; Ogata, Y.; Gomi, K.; Arai, T.; Fukae, M. Porcine enamel protein fractions contain transforming growth factor-β1. J. Periodontol. 2006, 77, 1688–1694. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamakawa, S.; Niwa, T.; Karakida, T.; Kobayashi, K.; Yamamoto, R.; Chiba, R.; Yamakoshi, Y.; Hosoya, N. Effects of Er:YAG and Diode Laser Irradiation on Dental Pulp Cells and Tissues. Int. J. Mol. Sci. 2018, 19, 2429. https://doi.org/10.3390/ijms19082429
Yamakawa S, Niwa T, Karakida T, Kobayashi K, Yamamoto R, Chiba R, Yamakoshi Y, Hosoya N. Effects of Er:YAG and Diode Laser Irradiation on Dental Pulp Cells and Tissues. International Journal of Molecular Sciences. 2018; 19(8):2429. https://doi.org/10.3390/ijms19082429
Chicago/Turabian StyleYamakawa, Shunjiro, Takahiko Niwa, Takeo Karakida, Kazuyuki Kobayashi, Ryuji Yamamoto, Risako Chiba, Yasuo Yamakoshi, and Noriyasu Hosoya. 2018. "Effects of Er:YAG and Diode Laser Irradiation on Dental Pulp Cells and Tissues" International Journal of Molecular Sciences 19, no. 8: 2429. https://doi.org/10.3390/ijms19082429
APA StyleYamakawa, S., Niwa, T., Karakida, T., Kobayashi, K., Yamamoto, R., Chiba, R., Yamakoshi, Y., & Hosoya, N. (2018). Effects of Er:YAG and Diode Laser Irradiation on Dental Pulp Cells and Tissues. International Journal of Molecular Sciences, 19(8), 2429. https://doi.org/10.3390/ijms19082429