Unraveling the Molecular Mechanism of Immunosenescence in Drosophila
Abstract
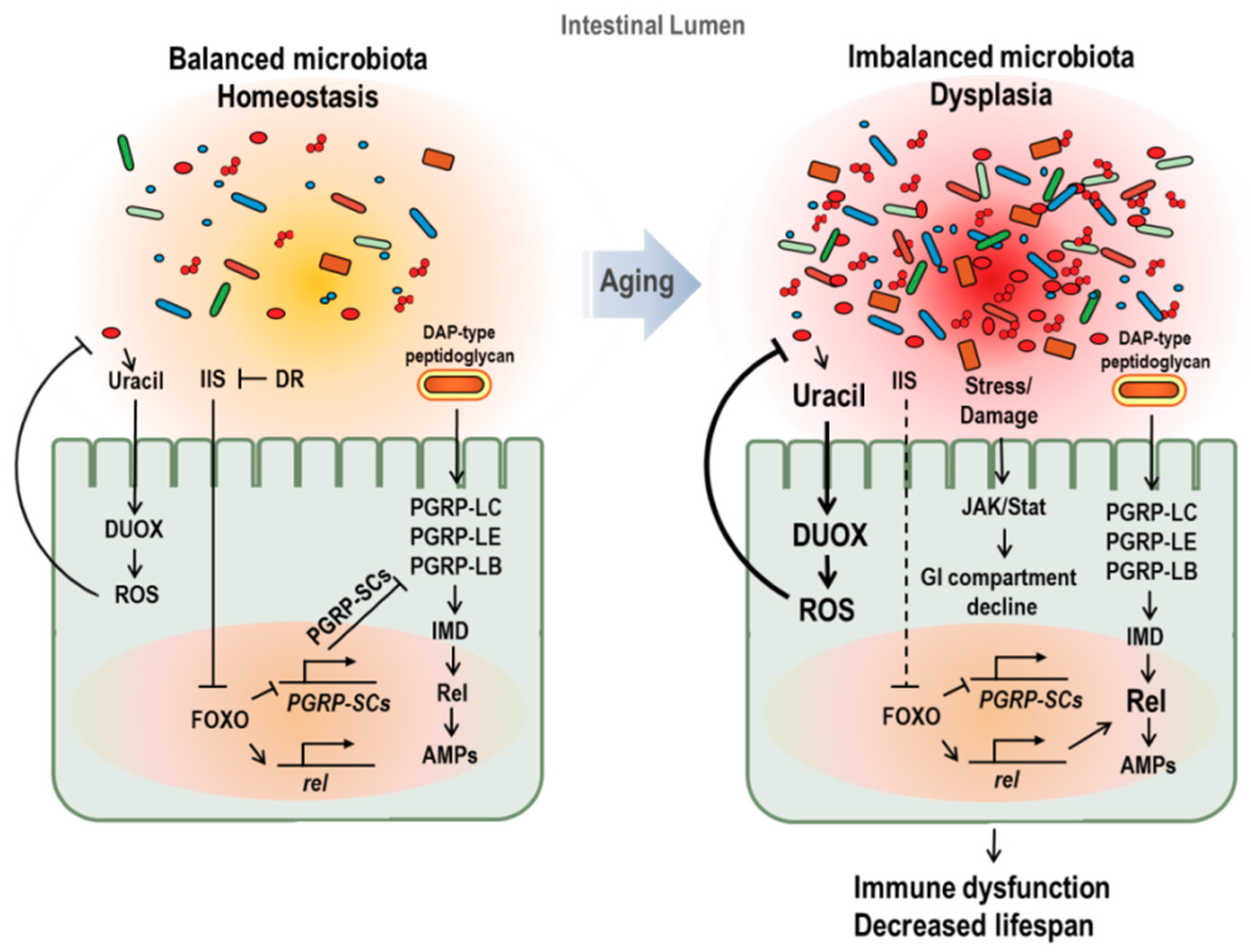
:1. Introduction
2. Innate Immunity in Drosophila
2.1. Innate Immune Signaling in Drosophila
2.2. Immune Senescence in Drosophila
2.3. Microbial Load Increase with Age
2.4. Increased Amp Production and Aging: Consequence or Cause?
3. Possible Mechanisms of Immunosenescence in Drosophila
3.1. Changes in Insulin/IGF-1 Signaling
3.2. Hormones (JH, 20E)
3.3. Degeneration of Feedback System
4. Emerging Role of Microbiota in the Regulation of Immunity and Aging in Drosophila
4.1. Effect of Commensal Bacteria on Development and Host Resistance
4.2. Effect of Gut Microbiota on Lifespan
4.3. Age-Dependent Changes in Gut Bacteria Populations
4.4. Changes in Intestinal Immunity and Physiology by Age
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IIS | insulin/IGF signaling |
| AMP | antimicrobial peptides |
| DR | dietary restriction |
| 20E | 20-hydroxyecdysone |
| JH | juvenile Hormone |
| FOXO | forkhead box O |
| PGRP | peptidoglycan recognition protein |
| DUOX | dual oxidase |
References
- Miller, R.A. The aging immune system: Primer and prospectus. Science 1996, 273, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.P. Aging of the immune system: How much can the adaptive immune system adapt? Immunity 2006, 24, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Pawelec, G.; Tarazona, R. Aging and innate immunity. Immunity 2006, 24, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Silverman, N.; Maniatis, T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001, 15, 2321–2342. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, M.; Rockwell, J.; Engleman, C.; Gewirtz, A.; Katz, J.; Sambhara, S. Cutting edge: Impaired Toll-like receptor expression and function in aging. J. Immunol. 2002, 169, 4697–4701. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Castillo, J.C. Molecular mechanisms of aging and immune system regulation in Drosophila. Int. J. Mol. Sci. 2012, 13, 9826–9844. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Seroude, L.; Benzer, S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 1998, 282, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Khazaeli, A.A.; Curtsinger, J.W. Chaperoning extended life. Nature 1997, 390, 30. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R.A. C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Chien, S.A.; Priest, N.K. Negligible Senescence during Reproductive Dormancy in Drosophila melanogaster. Am. Nat. 2001, 158, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Bartke, A.; Antebi, A. The endocrine regulation of aging by insulin-like signals. Science 2003, 299, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.N.; Shaposhnikov, M.V.; Sadritdinova, A.F.; Kudryavtseva, A.V.; Moskalev, A.A. Basic mechanisms of longevity: A case study of Drosophila pro-longevity genes. Ageing Res. Rev. 2015, 24, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Mair, W.; Goymer, P.; Pletcher, S.D.; Partridge, L. Demography of dietary restriction and death in Drosophila. Science 2003, 301, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Harshman, L.G.; Zera, A.J. The cost of reproduction: The devil in the details. Trends Ecol. Evol. 2007, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, S.H.; Min, K.J. Insects as a model system for aging studies. Entomol. Res. 2014, 45, 1–8. [Google Scholar] [CrossRef]
- Hetru, C.; Troxler, L.; Hoffmann, J.A. Drosophila melanogaster antimicrobial defense. J. Infect. Dis. 2003, 187, S327–S334. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Rowe, D.C.; Barnes, B.J.; Caffrey, D.R.; Visintin, A.; Latz, E.; Monks, B.; Pitha, P.M.; Golenbock, D.T. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J. Exp. Med. 2003, 198, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Burns, K.; Hofmann, K.; Blancheteau, V.; Martinon, F.; Kelliher, M.; Tschopp, J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB. activation. Nat. Immunol. 2004, 5, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Garschall, K.; Flatt, T. The interplay between immunity and aging in Drosophila. F1000Research 2018, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Walker, D.W. Role of gut microbiota in aging-related health decline: Insights from invertebrate models. Cell. Mol. Life Sci. 2018, 75, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Seroude, L.; Brummel, T.; Kapahi, P.; Benzer, S. Spatio-temporal analysis of gene expression during aging in Drosophila melanogaster. Aging Cell 2002, 1, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, S.D.; Macdonald, S.J.; Marguerie, R.; Certa, U.; Stearns, S.C.; Goldstein, D.B.; Partridge, L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol. 2002, 12, 712–723. [Google Scholar] [CrossRef]
- Zerofsky, M.; Harel, E.; Silverman, N.; Tatar, M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 2005, 4, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Landis, G.N.; Abdueva, D.; Skvortsov, D.; Yang, J.; Rabin, B.E.; Carrick, J.; Tavare, S.; Tower, J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2004, 101, 7663–7668. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Webster, P.; Finkel, S.E.; Tower, J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007, 6, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sarup, P.; Sorensen, P.; Loeschcke, V. Flies selected for longevity retain a young gene expression profile. Age (Dordr) 2011, 33, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Karpac, J.; Tran, S.L.; Jasper, H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell 2014, 156, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Libert, S.; Chao, Y.; Chu, X.; Pletcher, S.D. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NF-κB signaling. Aging Cell 2006, 5, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.M.; Hwangbo, D.S.; Corby-Harris, V.; Promislow, D.E. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell 2007, 6, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, S.; Cheung, Y.Y.; Seroude, L. Functional analysis of the Drosophila immune response during aging. Aging Cell 2008, 7, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Nam, H.J.; Chung, H.Y.; Kim, N.D.; Ryu, J.H.; Lee, W.J.; Arking, R.; Yoo, M.A. Role of xanthine dehydrogenase and aging on the innate immune response of Drosophila. J. Am. Aging Assoc. 2001, 24, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lesser, K.J.; Paiusi, I.C.; Leips, J. Naturally occurring genetic variation in the age-specific immune response of Drosophila melanogaster. Aging Cell 2006, 5, 293–295. [Google Scholar] [CrossRef] [PubMed]
- DeVeale, B.; Brummel, T.; Seroude, L. Immunity and aging: The enemy within? Aging Cell 2004, 3, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Badinloo, M.; Nguyen, E.; Suh, W.; Alzahrani, F.; Castellanos, J.; Klichko, V.I.; Orr, W.C.; Radyuk, S.N. Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues. Arch. Insect. Biochem. Physiol. 2018, e21464. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.R.; Parikh, H.; Park, Y. Stress resistance and lifespan enhanced by downregulation of antimicrobial peptide genes in the Imd pathway. Aging (Albany NY) 2018, 10, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loch, G.; Zinke, I.; Mori, T.; Carrera, P.; Schroer, J.; Takeyama, H.; Hoch, M. Antimicrobial peptides extend lifespan in Drosophila. PLoS ONE 2017, 12, e0176689. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.J.; Piper, M.D.; Ikeya, T.; Bass, T.M.; Jacobson, J.; Driege, Y.; Martinez, P.; Hafen, E.; Withers, D.J.; Leevers, S.J.; et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA 2005, 102, 3105–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronke, S.; Clarke, D.F.; Broughton, S.; Andrews, T.D.; Partridge, L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010, 6, e1000857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alic, N.; Hoddinott, M.P.; Vinti, G.; Partridge, L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell 2011, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Wessells, R.J.; Fitzgerald, E.; Cypser, J.R.; Tatar, M.; Bodmer, R. Insulin regulation of heart function in aging fruit flies. Nat. Genet. 2004, 36, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Libert, S.; Chao, Y.; Zwiener, J.; Pletcher, S.D. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol. Immunol. 2008, 45, 810–817. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.; Yadav, S.; Shokal, U.; Kenney, E.; Cooper, D.; Eleftherianos, I. The insulin receptor substrate Chico regulates antibacterial immune function in Drosophila. Immun. Ageing 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Slack, C.; Giannakou, M.E.; Foley, A.; Goss, M.; Partridge, L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell 2011, 10, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Loch, G.; Beyer, M.; Zinke, I.; Aschenbrenner, A.C.; Carrera, P.; Inhester, T.; Schultze, J.L.; Hoch, M. FOXO-dependent regulation of innate immune homeostasis. Nature 2010, 463, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M. Brown University, Providence, RI, USA. 2018; unpublished work. [Google Scholar]
- Flatt, T.; Tu, M.P.; Tatar, M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays 2005, 27, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.S.; Richard, D.S.; Applebaum, S.W.; Ma, M.; Gilbert, L.I. Photoperiodic diapause in Drosophila melanogaster involves a block to the juvenile hormone regulation of ovarian maturation. Gen. Comp. Endocrinol. 1990, 79, 174–184. [Google Scholar] [CrossRef]
- Tu, M.P.; Yin, C.M.; Tatar, M. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen. Comp. Endocrinol. 2005, 142, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Bai, H.; Dolezal, A.G.; Amdam, G.; Tatar, M. Juvenile hormone regulation of Drosophila aging. BMC Biol. 2013, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.F.; Shih, C.; Mack, A.; Benzer, S. Steroid control of longevity in Drosophila melanogaster. Science 2003, 299, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, H.; Battisti, V.; Trannoy, S.; Lasbleiz, C.; Pret, A.M.; Monnier, V. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech. Ageing Dev. 2009, 130, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Beckstead, R.B.; Lam, G.; Thummel, C.S. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 2005, 6, R99. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Guo, E.; Diao, Y.; Zhou, S.; Peng, Q.; Cao, Y.; Ling, E.; Li, S. Genome-wide regulation of innate immunity by juvenile hormone and 20-hydroxyecdysone in the Bombyx fat body. BMC Genom. 2010, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.; Richards, G. Ecdysone and insect immunity: The maturation of the inducibility of the diptericin gene in Drosophila larvae. Insect Biochem. Mol. Biol. 1996, 26, 155–160. [Google Scholar] [CrossRef]
- Rus, F.; Flatt, T.; Tong, M.; Aggarwal, K.; Okuda, K.; Kleino, A.; Yates, E.; Tatar, M.; Silverman, N. Ecdysone triggered PGRP-LC expression controls Drosophila innate immunity. EMBO J. 2013, 32, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Flatt, T.; Heyland, A.; Rus, F.; Porpiglia, E.; Sherlock, C.; Yamamoto, R.; Garbuzov, A.; Palli, S.R.; Tatar, M.; Silverman, N. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J. Exp. Biol. 2008, 211, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Vainikka, A.; Kortet, R. The role of juvenile hormone in immune function and pheromone production trade-offs: A test of the immunocompetence handicap principle. Proc. Biol. Sci. 2003, 270, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Simoes, Z.L.; Hagen, A.; Norberg, K.; Schroder, K.; Mikkelsen, O.; Kirkwood, T.B.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.M. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev. Biol. 1982, 93, 73–82. [Google Scholar] [CrossRef]
- Zheng, W.; Rus, F.; Hernandez, A.; Kang, P.; Goldman, W.; Silverman, N.; Tatar, M. Dehydration triggers ecdysone-mediated recognition-protein priming and elevated anti-bacterial immune responses in Drosophila Malpighian tubule renal cells. BMC Biol. 2018, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Silverman, N. Positive and negative regulation of the Drosophila immune response. BMB Rep. 2008, 41, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.S.; Enslen, H.; Hu, X.; Meng, X.; Wu, I.H.; Barrett, T.; Davis, R.J.; Ip, Y.T. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol. Cell. Biol. 1998, 18, 3527–3539. [Google Scholar] [CrossRef] [PubMed]
- Vrailas-Mortimer, A.; del Rivero, T.; Mukherjee, S.; Nag, S.; Gaitanidis, A.; Kadas, D.; Consoulas, C.; Duttaroy, A.; Sanyal, S. A muscle-specific p38 MAPK/Mef2/MnSOD pathway regulates stress, motor function, and life span in Drosophila. Dev. Cell 2011, 21, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Youngman, M.J.; Rogers, Z.N.; Kim, D.H. A decline in p38 MAPK signaling underlies immunosenescence in Caenorhabditis elegans. PLoS Genet. 2011, 7, e1002082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harries, L.W. MicroRNAs as Mediators of the Ageing Process. Genes 2014, 5, 656–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalaei-andabili, S.; Zare-Bidoki, A.; Rezaei, N. The role of microRNAs in immunosenescence process. In Immunology of Aging; Massoud, A., Rezaei, N., Eds.; Springer: Berlin, Germany, 2014; pp. 211–217. [Google Scholar]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of bic/microRNA-155 for normal immune function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Seeger, T.; Haffez, F.; Fischer, A.; Koehl, U.; Leistner, D.M.; Seeger, F.H.; Boon, R.A.; Zeiher, A.M.; Dimmeler, S. Immunosenescence-associated microRNAs in age and heart failure. Eur. J. Heart Fail. 2013, 15, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, D. Aging and immunosenescence in invertebrates. Invertebr. Surviv. J. 2012, 9, 102–109. [Google Scholar]
- Liu, N.; Landreh, M.; Cao, K.; Abe, M.; Hendriks, G.J.; Kennerdell, J.R.; Zhu, Y.; Wang, L.S.; Bonini, N.M. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature 2012, 482, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Fullaondo, A.; Lee, S.Y. Identification of putative miRNA involved in Drosophila melanogaster immune response. Dev. Comp. Immunol. 2012, 36, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.K.; Hyun, S. Conserved microRNA miR-8 in fat body regulates innate immune homeostasis in Drosophila. Dev. Comp. Immunol. 2012, 37, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Garbuzov, A.; Tatar, M. Hormonal regulation of Drosophila microRNA let-7 and miR-125 that target innate immunity. Fly (Austin) 2010, 4, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.N.; Ng, P.; Douglas, A.E. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 2011, 13, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.E.; Fischer, C.N.; Miles, J.; Handelsman, J. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. mBio 2013, 4, e00860-13. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Chaston, J.M.; Douglas, A.E. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013, 7, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Brummel, T.; Ching, A.; Seroude, L.; Simon, A.F.; Benzer, S. Drosophila lifespan enhancement by exogenous bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 12974–12979. [Google Scholar] [CrossRef] [PubMed]
- Ridley, E.V.; Wong, A.C.; Westmiller, S.; Douglas, A.E. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS ONE 2012, 7, e36765. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W.; et al. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, R.; Deshpande, S.A.; Bruce, K.D.; Mak, E.M.; Ja, W.W. Microbes Promote Amino Acid Harvest to Rescue Undernutrition in Drosophila. Cell Rep. 2015, 10, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.L.; Zhang, V.; Lamberti, L.; Jones, E.W.; Obadia, B.; Gavryushkin, A.; Carlson, J.M.; Beerenwinkel, N.; Ludington, W.B. High-dimensional microbiome interactions shape host fitness. BioRxiv 2017. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, S.H.; Lee, H.Y.; Bai, J.Y.; Nam, Y.D.; Bae, J.W.; Lee, D.G.; Shin, S.C.; Ha, E.M.; Lee, W.J. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 2008, 319, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lee, H.J.; Jeong, S.E.; Jeon, C.O.; Hyun, S. Comparative Analysis of Drosophila melanogaster Gut Microbiota with Respect to Host Strain, Sex, and Age. Microb. Ecol. 2017, 74, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.M.; Werner, T.; Stoven, S.; Hultmark, D.; Anderson, K.V. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 2002, 296, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, S.H.; Kim, E.K.; Ha, E.M.; You, H.; Kim, B.; Kim, M.J.; Kwon, Y.; Ryu, J.H.; Lee, W.J. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell 2013, 153, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Zheng, Y. Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell 2014, 159, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Erkosar, B.; Leulier, F. Transient adult microbiota, gut homeostasis and longevity: Novel insights from the Drosophila model. FEBS Lett. 2014, 588, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Karpac, J.; Supoyo, S.; Degennaro, M.; Lehmann, R.; Jasper, H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010, 6, e1001159. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef] [PubMed]
- Karpac, J.; Biteau, B.; Jasper, H. Misregulation of an adaptive metabolic response contributes to the age-related disruption of lipid homeostasis in Drosophila. Cell Rep. 2013, 4, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jasper, H. Gastrointestinal stem cells in health and disease: From flies to humans. Dis. Model. Mech. 2016, 9, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Catterson, J.H.; Khericha, M.; Dyson, M.C.; Vincent, A.J.; Callard, R.; Haveron, S.M.; Rajasingam, A.; Ahmad, M.; Partridge, L. Short-Term, Intermittent Fasting Induces Long-Lasting Gut Health and TOR-Independent Lifespan Extension. Curr. Biol. 2018, 28, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.C.; Khericha, M.; Dobson, A.J.; Bolukbasi, E.; Rattanavirotkul, N.; Partridge, L. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife 2016, 5, e10956. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.-J.; Tatar, M. Unraveling the Molecular Mechanism of Immunosenescence in Drosophila. Int. J. Mol. Sci. 2018, 19, 2472. https://doi.org/10.3390/ijms19092472
Min K-J, Tatar M. Unraveling the Molecular Mechanism of Immunosenescence in Drosophila. International Journal of Molecular Sciences. 2018; 19(9):2472. https://doi.org/10.3390/ijms19092472
Chicago/Turabian StyleMin, Kyung-Jin, and Marc Tatar. 2018. "Unraveling the Molecular Mechanism of Immunosenescence in Drosophila" International Journal of Molecular Sciences 19, no. 9: 2472. https://doi.org/10.3390/ijms19092472





