Identification of Oxidative Stress in Red Blood Cells with Nanoscale Chemical Resolution by Infrared Nanospectroscopy
Abstract
:1. Introduction
2. Results
2.1. Nanoscale AFM-IR of Individual RBCs
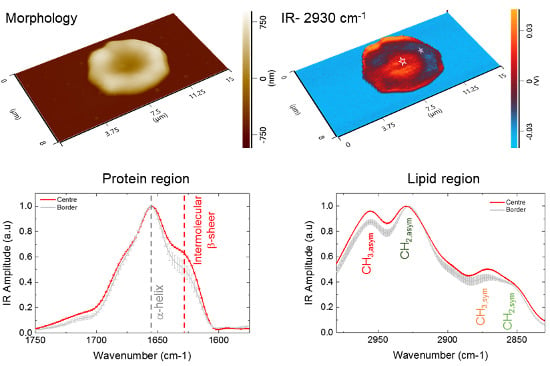
2.2. Sub-cellular Investigation of Individual RBCs
2.3. Comparison of Biconcave and Echinocyte
2.4. Membrane Peroxidation of RBCs
3. Discussion
4. Materials and Methods
4.1. RBC Sample Preparation
4.2. AFM-IR Measurements
Author Contributions
Funding
Conflicts of Interest
References
- Girasole, M.; Pompeo, G.; Cricenti, A.; Longo, G.; Boumis, G.; Bellelli, A.; Amiconi, S. The how, when, and why of the aging signals appearing on the human erythrocyte membrane: An atomic force microscopy study of surface roughness. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and function in native and pathological erythrocytes: A quantitative view from the nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Wu, Z.J.; Mehrishi, J.; Huang, B.T.; Chen, X.Y.; Zheng, X.J.; Liu, W.J.; Luo, M. Human red blood cell aging: Correlative changes in surface charge and cell properties. J. Cell. Mol. Med. 2011, 15, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Chin-Yee, I.; Arya, N.; d’Almeida, M.S. The red cell storage lesion and its implication for transfusion. Transfus. Sci. 1997, 18, 447–458. [Google Scholar] [CrossRef]
- Bosman, G.J.C.G.M.; Werre, J.M.; Willekens, F.L.A.; Novotný, V.M.J. Erythrocyte ageing in vivo and in vitro: Structural aspects and implications for transfusion. Transfus. Med. 2008, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structural, morphological and nanomechanical characterisation of intermediate states in the ageing of erythrocytes. J. Mol. Recognit. 2012, 25, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Adamson, J.W. New blood, old blood, or no blood? N. Engl. J. Med. 2008, 358, 1295–1296. [Google Scholar] [CrossRef] [PubMed]
- Gov, N.S.; Safran, S.A. Red blood cell membrane fluctuations and shape controlled by atp-induced cytoskeletal defects. Biophys. J. 2005, 88, 1859–1874. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Pushkaran, S.; Li, L.; An, X.; Zheng, Y.; Mohandas, N.; Joiner, C.H.; Kalfa, T.A. Altered phosphorylation of cytoskeleton proteins in sickle red blood cells: The role of protein kinase c, rac gtpases, and reactive oxygen species. Blood Cells Mol. Dis. 2010, 45, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, N.; Chasis, J.A. Red blood cell deformability, membrane material properties and shape: Regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin. Hematol. 1993, 30, 171–192. [Google Scholar] [PubMed]
- Dinarelli, S.; Longo, G.; Dietler, G.; Francioso, A.; Mosca, L.; Pannitteri, G.; Boumis, G.; Bellelli, A.; Girasole, M. Erythrocyte’s aging in microgravity highlights how environmental stimuli shape metabolism and morphology. Sci. Rep. 2018, 8, 5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rettig, M.P.; Low, P.S.; Gimm, J.A.; Mohandas, N.; Wang, J.; Christian, J.A. Evaluation of biochemical changes during in vivo erythrocyte senescence in the dog. Blood 1999, 93, 376–384. [Google Scholar] [PubMed]
- Forsyth, A.M.; Wan, J.; Owrutsky, P.D.; Abkarian, M.; Stone, H.A. Multiscale approach to link red blood cell dynamics, shear viscosity, and atp release. Proc. Natl. Acad. Sci. USA 2011, 108, 10986–10991. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, E.; Chernysh, A.; Moroz, V.; Sergunova, V.; Gudkova, O.; Manchenko, E. Morphology, membrane nanostructure and stiffness for quality assessment of packed red blood cells. Sci. Rep. 2017, 7, 7846. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, E.K.; Chernysh, A.M.; Moroz, V.V.; Kuzovlev, A.N. Analysis of nanostructure of red blood cells membranes by space fourier transform of afm images. Micron 2013, 44, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Tuvia, S.; Levin, S.; Bitler, A.; Korenstein, R. Mechanical fluctuations of the membrane-skeleton are dependent on f-actin atpase in human erythrocytes. J. Cell Biol. 1998, 141, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Carelli-Alinovi, C.; Dinarelli, S.; Girasole, M.; Misiti, F. Vascular dysfunction-associated with alzheimer’s disease. Clin. Hemorheol. Microcirc. 2016, 64, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Fuller, J. Oxidative stress in erythrocytes. Comp. Haematol. Int. 1996, 6, 24–31. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 1999, 34, 1–104. [Google Scholar] [CrossRef] [Green Version]
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared spectroscopy of membrane lipids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1074–1081. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Wiley Online Library: Hoboken, NJ, USA, 2005; pp. 1–208. [Google Scholar]
- Ruggeri, F.S.; Habchi, J.; Cerreta, A.; Dietler, G. Afm-based single molecule techniques: Unraveling the amyloid pathogenic species. Curr. Pharm. Des. 2016, 22, 3950–3970. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M. Local infrared microspectroscopy with subwavelength spatial resolution with an atomic force microscope tip used as a photothermal sensor. Opt. Lett. 2005, 30, 2388–2390. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, A.; Prater, C.B. Afm-ir: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, A.; Prater, C.B.; Hu, Q.; Chase, D.B.; Rabolt, J.F.; Marcott, C. Afm-ir: Combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 2012, 66, 1365–1384. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, B.; Holland, G.; Centrone, A. Chemical imaging beyond the diffraction limit: Experimental validation of the ptir technique. Small 2013, 9, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guaita, D.; Kochan, K.; Batty, M.; Doerig, C.; Garcia-Bustos, J.; Espinoza, S.; McNaughton, D.; Heraud, P.; Wood, B.R. Multispectral atomic force microscopy-infrared nano-imaging of malaria infected red blood cells. Anal. Chem. 2018, 90, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Volpatti, L.R.; Shimanovich, U.; Ruggeri, F.S.; Bolisetty, S.; Muller, T.; Mason, T.O.; Michaels, T.C.T.; Mezzenga, R.; Dietler, G.; Knowles, T.P.J. Micro- and nanoscale hierarchical structure of core-shell protein microgels. J. Mater. Chem. B 2016, 4, 7989–7999. [Google Scholar] [CrossRef]
- Qamar, S.; Wang, G.; Randle, S.J.; Ruggeri, F.S.; Varela, J.A.; Lin, J.Q.; Phillips, E.C.; Miyashita, A.; Williams, D.; Ströhl, F.; et al. Fus phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell 2018, 173, 720–734.e715. [Google Scholar] [CrossRef] [PubMed]
- Ramer, G.; Ruggeri, F.S.; Levin, A.; Knowles, T.P.J.; Centrone, A. Determination of polypeptide conformation with nanoscale resolution in water. ACS Nano 2018, 12, 6612–6619. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Vieweg, S.; Cendrowska, U.; Longo, G.; Chiki, A.; Lashuel, H.A.; Dietler, G. Nanoscale studies link amyloid maturity with polyglutamine diseases onset. Sci. Rep. 2016, 6, 31155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galante, D.; Ruggeri, F.S.; Dietler, G.; Pellistri, F.; Gatta, E.; Corsaro, A.; Florio, T.; Perico, A.; D’Arrigo, C. A critical concentration of n-terminal pyroglutamylated amyloid beta drives the misfolding of ab1-42 into more toxic aggregates. Int. J. Biochem. Cell Biol. 2016, 79, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Mayet, C.; Dazzi, A.; Prazeres, R.; Allot, F.; Glotin, F.; Ortega, J.M. Sub-100 nm ir spectromicroscopy of living cells. Opt. Lett. 2008, 33, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M.; Al-Sawaftah, M.; de Frutos, M. Chemical mapping of the distribution of viruses into infected bacteria with a photothermal method. Ultramicroscopy 2008, 108, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Mayet, C.; Dazzi, A.; Prazeres, R.; Ortega, J.M.; Jaillard, D. In situ identification and imaging of bacterial polymer nanogranules by infrared nanospectroscopy. Analyst 2010, 135, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, L.; Giliberti, V.; Rosa, A.; Ortolani, M.; Bonamore, A.; Baiocco, P.; Kjoller, K.; Calvani, P.; Nucara, A. Mapping the amide i absorption in single bacteria and mammalian cells with resonant infrared nanospectroscopy. Nanotechnology 2016, 27, 075101. [Google Scholar] [CrossRef] [PubMed]
- Marcott, C.; Lo, M.; Kjoller, K.; Fiat, F.; Baghdadli, N.; Balooch, G.; Luengo, G.S. Localization of human hair structural lipids using nanoscale infrared spectroscopy and imaging. Appl. Spectrosc. 2014, 68, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Policar, C.; Waern, J.B.; Plamont, M.A.; Clede, S.; Mayet, C.; Prazeres, R.; Ortega, J.M.; Vessieres, A.; Dazzi, A. Subcellular ir imaging of a metal-carbonyl moiety using photothermally induced resonance. Angew. Chem. Int. Ed. Engl. 2011, 50, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Gourion-Arsiquaud, S.; Marcott, C.; Hu, Q.; Boskey, A.L. Studying variations in bone composition at nano-scale resolution: A preliminary report. Calcif. Tissue Int. 2014, 95, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Dinarelli, S.; Longo, G.; Krumova, S.; Todinova, S.; Danailova, A.; Taneva, S.G.; Lenzi, E.; Mussi, V.; Girasole, M. Insights into the morphological pattern of erythrocytes’ aging: Coupling quantitative afm data to microcalorimetry and raman spectroscopy. J. Mol. Recognit. 2018, e2732. [Google Scholar] [CrossRef] [PubMed]
- Giliberti, V.; Baldassarre, L.; Rosa, A.; de Turris, V.; Ortolani, M.; Calvani, P.; Nucara, A. Protein clustering in chemically stressed hela cells studied by infrared nanospectroscopy. Nanoscale 2016, 8, 17560–17567. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Deleris, G. Oxidative stress effects on erythrocytes determined by ft-ir spectrometry. Analyst 2004, 129, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Oleszko, A.; Olsztyńska-Janus, S.; Walski, T.; Grzeszczuk-Kuć, K.; Bujok, J.; Gałecka, K.; Czerski, A.; Witkiewicz, W.; Komorowska, M. Application of FT-IR-atr spectroscopy to determine the extent of lipid peroxidation in plasma during haemodialysis. BioMed Res. Int. 2015, 2015, 245607. [Google Scholar] [CrossRef] [PubMed]
- Vileno, B.; Jeney, S.; Sienkiewicz, A.; Marcoux, P.R.; Miller, L.M.; Forro, L. Evidence of lipid peroxidation and protein phosphorylation in cells upon oxidative stress photo-generated by fullerols. Biophys. Chem. 2010, 152, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sills, R.H.; Moore, D.J.; Mendelsohn, R. Erythrocyte peroxidation: Quantitation by fourier transform infrared spectroscopy. Anal. Biochem. 1994, 218, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Dinarelli, S.; Girasole, M.; Longo, G. Methods for atomic force microscopy of biological and living specimens. In Nanoscale Imaging: Methods and Protocols; Lyubchenko, Y.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 529–539. [Google Scholar]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggeri, F.S.; Marcott, C.; Dinarelli, S.; Longo, G.; Girasole, M.; Dietler, G.; Knowles, T.P.J. Identification of Oxidative Stress in Red Blood Cells with Nanoscale Chemical Resolution by Infrared Nanospectroscopy. Int. J. Mol. Sci. 2018, 19, 2582. https://doi.org/10.3390/ijms19092582
Ruggeri FS, Marcott C, Dinarelli S, Longo G, Girasole M, Dietler G, Knowles TPJ. Identification of Oxidative Stress in Red Blood Cells with Nanoscale Chemical Resolution by Infrared Nanospectroscopy. International Journal of Molecular Sciences. 2018; 19(9):2582. https://doi.org/10.3390/ijms19092582
Chicago/Turabian StyleRuggeri, Francesco S., Curtis Marcott, Simone Dinarelli, Giovanni Longo, Marco Girasole, Giovanni Dietler, and Tuomas P. J. Knowles. 2018. "Identification of Oxidative Stress in Red Blood Cells with Nanoscale Chemical Resolution by Infrared Nanospectroscopy" International Journal of Molecular Sciences 19, no. 9: 2582. https://doi.org/10.3390/ijms19092582