ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy
Abstract
:1. Introduction
2. Results
2.1. ATP Induces an Increase in [Ca2+]i by Activating the P2Y2 Receptor and Downstream IP3R
2.2. ATP Activates mTOR via the P2Y2 Receptor/PLC/IP3R Pathway In Vitro
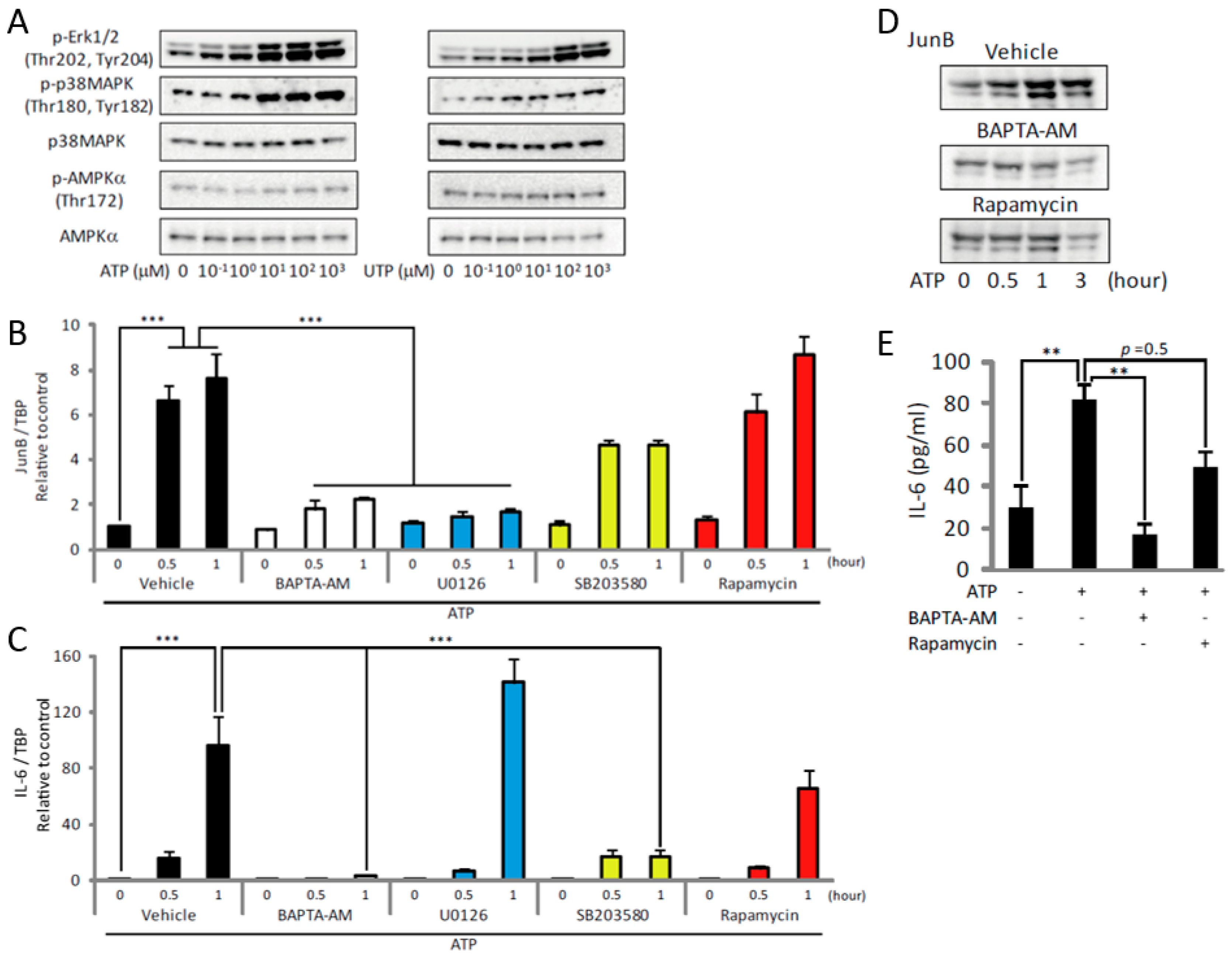
2.3. ATP Upregulates JunB and IL-6 by Activating MAPKs and mTOR
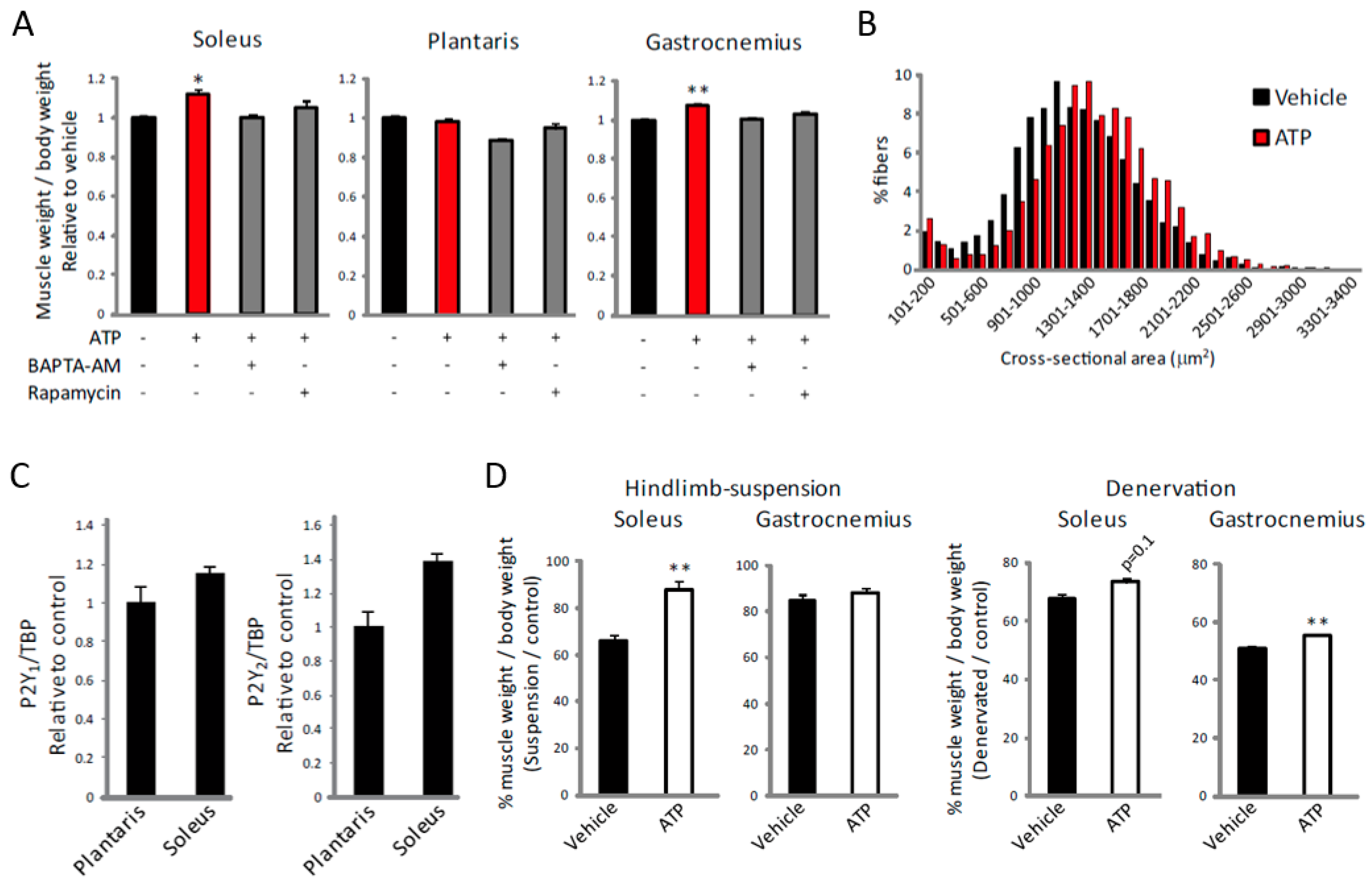
2.4. ATP Induces an Increase in [Ca2+]i Specifically in Slow Muscles
2.5. ATP Induces Muscle Hypertrophy by Regulating [Ca2+]i
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
4.3. C2C12 Cell Culture
4.4. Gene Knockdown by RNA Interference
4.5. Retrovirus-Mediated Gene Transfer
4.6. Intracellular Ca2+ Level Measurement
4.7. Western Blot Analysis
4.8. RNA Preparation and RT-qPCR
4.9. Exercise Model
4.10. ELISA
4.11. Hindlimb-Suspension and Denervation Surgery
4.12. Histological and Immunohistological Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPKα | AMP-activated protein kinase α |
| EDL | extensor digitorum longus |
| IP3R | inositol 1,4,5-trisphosphate receptor |
| IGF-1 | linear dichroism |
| IL-6 | interleukin-6 |
| [Ca2+]i | intracellular Ca2+ level |
| mTOR | mammalian target of rapamycin |
| MAPK | mitogen-activated protein kinase |
| nNOS | neuronal nitric oxide synthase |
| PI3K | phosphatidylinositol 3-kinase |
| PLC | phospholipase C |
| SR | sarcoplasmic reticulum |
| TBP | TATA-binding protein |
| XeC | xestospongin C |
References
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by Pl(3)K/Alt/mTOR and Pl(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev. Physiol. Biochem. Pharmacol. 2014, 166, 43–95. [Google Scholar] [PubMed]
- Spangenburg, E.E.; Le Roith, D.; Ward, C.W.; Bodine, S.C. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J. Physiol. 2008, 586, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philp, A.; Hamilton, D.L.; Baar, K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J. Appl. Physiol. 2011, 110, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornberger, T.A.; Stuppard, R.; Conley, K.E.; Fedele, M.J.; Fiorotto, M.L.; Chin, E.R.; Esser, K.A. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem. J. 2004, 380, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Mccarthy, J.J.; Fedele, M.J.; Esser, K.A. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J. Physiol. 2011, 589, 1831–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 2013, 19, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Capsaicin mimics mechanical load-induced intracellular signaling events. Channels 2013, 7, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buvinic, S.; Almarza, G.; Bustamante, M.; Casas, M.; López, J.; Riquelme, M.; Sáez, J.C.; Huidobro-Toro, J.P.; Jaimovich, E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 2009, 284, 34490–34505. [Google Scholar] [CrossRef] [PubMed]
- Forrester, T.; Lind, A.R. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J. Physiol. 1969, 204, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; King, N.C.; Sinoway, L.I. ATP concentrations and muscle tension increase linearly with muscle contraction. J. Appl. Physiol. 2003, 95, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, K.; Seta, R.; Shimizu, T.; Shinkai, S.; Calderwood, S.K.; Nakazato, K.; Takahashi, K. Plasma adenosine triphosphate and heat shock protein 72 concentrations after aerobic and eccentric exercise. Exerc. Immunol. Rev. 2011, 17, 136–149. [Google Scholar] [PubMed]
- Fumagalli, M.; Lecca, D.; Abbracchio, M.P.; Ceruti, S. Pathophysiological role of purines and pyrimidines in neurodevelopment: Unveiling new pharmacological approaches to congenital brain diseases. Front. Pharmacol. 2017, 8, 941. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Verkhratsky, A. Receptors for Purines and Pyrimidines. In Purinergic Signalling and the Nervous System; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-28862-3. [Google Scholar]
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Front. Oncol. 2017, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Tallini, Y.N.; Ohkura, M.; Choi, B.-R.; Ji, G.; Imoto, K.; Doran, R.; Lee, J.; Plan, P.; Wilson, J.; Xin, H.-B.; et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc. Natl. Acad. Sci. USA 2006, 103, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.H.; Nelemans, A.; van den Akker, J.; den Hertog, A. The nucleotide receptors on mouse C2C12 myotubes. Br. J. Pharmacol. 1992, 106, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, R.H.; Duin, M.; den Hertog, A.; Nelemans, A. Characterization of P2-purinoceptor mediated cyclic AMP formation in mouse C2C12 myotubes. Br. J. Pharmacol. 1993, 110, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, R.H.; Duin, M.; den Hertog, A.; Nelemans, A. Activation of the phospholipase C pathway by ATP is mediated exclusively through nucleotide type P2-purinoceptors in C2C12 myotubes. Br. J. Pharmacol. 1993, 110, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, K.K.Y.; Siow, N.L.; Choi, R.C.Y.; Tsim, K.W.K. ATP potentiates the formation of AChR aggregate in the co-culture of NG108-15 cells with C2C12 myotubes. FEBS Lett. 2005, 579, 2469–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deli, T.; Tóth, B.I.; Czifra, G.; Szappanos, H.; Bíró, T.; Csernoch, L. Differences in purinergic and voltage-dependent signalling during protein kinase Cα overexpression- and culturing-induced differentiation of C2C12 myoblasts. J. Muscle Res. Cell Motil. 2006, 27, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Rigault, C.; Bernard, A.; Georges, B.; Kandel, A.; Pfützner, E.; Le Borgne, F.; Demarquoy, J. Extracellular ATP increases L-carnitine transport and content in C2C12 cells. Pharmacology 2008, 81, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Banachewicz, W.; Supłat, D.; Krzemiński, P.; Pomorski, P.; Barańska, J. P2 nucleotide receptors on C2C12 satellite cells. Purinergic Signal. 2005, 1, 249. [Google Scholar] [CrossRef] [PubMed]
- Lustig, K.D.; Shiau, A.K.; Brake, A.J.; Julius, D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. USA 1993, 90, 5113–5117. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Yoshikawa, F.; Hishida, A.; Furuichi, T.; Mikoshiba, K. A novel recombinant hyperaffinity inositol 1,4,5-trisphosphate (IP3) absorbent traps IP3, resulting in specific inhibition of IP3-mediated calcium signaling. J. Biol. Chem. 2002, 277, 8106–8113. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.J.; Bootman, M.D.; Roderick, H.L. Cell signalling: IP3 receptors channel calcium into cell death. Curr. Biol. 2004, 14, R933–R935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimobayashi, M.; Hall, M.N. Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Fernandez-Verdejo, R.; Jaimovich, E.; Buvinic, S. Electrical stimulation induces IL-6 in skeletal muscle through extracellular ATP by activating Ca2+ signals and an IL-6 autocrine loop. AJP Endocrinol. Metab. 2014, 306, E869–E882. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Raffaello, A.; Milan, G.; Masiero, E.; Carnio, S.; Lee, D.; Lanfranchi, G.; Goldberg, A.L.; Sandri, M. JunB transcription factor maintains skeletal muscle mass and promotes hypertrophy. J. Cell Biol. 2010, 191, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 Is an Essential Regulator of Satellite Cell-Mediated Skeletal Muscle Hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, D.J.; Parise, G.; Melov, S.; Safdar, A.; Tarnopolsky, M.A. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005, 19, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Akerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Del Piccolo, P.; Rodriguez, L.; Hernandez Gonzalez, V.-H.; Agatea, L.; Solagna, F.; Mammano, F.; Pozzan, T.; Schiaffino, S. No evidence for inositol 1,4,5-trisphosphate-dependent Ca2+ release in isolated fibers of adult mouse skeletal muscle. J. Gen. Physiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vegas, A.; Campos, C.A.; Contreras-Ferrat, A.; Casas, M.; Buvinic, S.; Jaimovich, E.; Espinosa, A. ROS production via P2Y1-PKC-NOX2 is triggered by extracellular ATP after electrical stimulation of skeletal muscle cells. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Buvinic, S.; Jaimovich, E. ATP signaling in skeletal muscle: From fiber plasticity to regulation of metabolism. Exerc. Sport Sci. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Finch, E.A.; Graham, V.; Zhang, Z.-S.; Ding, J.-D.; Burch, J.; Oh-hora, M.; Rosenberg, P. STIM1-Ca2+ Signaling Is Required for the Hypertrophic Growth of Skeletal Muscle in Mice. Mol. Cell. Biol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Schakman, O.; Louis, P.; Ruegg, U.T.; Dietrich, A.; Birnbaumer, L.; Gailly, P. Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. J. Biol. Chem. 2012. [Google Scholar] [CrossRef] [PubMed]
- Gulati, P.; Gaspers, L.D.; Dann, S.G.; Joaquin, M.; Nobukuni, T.; Natt, F.; Kozma, S.C.; Thomas, A.P.; Thomas, G. Amino Acids Activate mTOR Complex 1 via Ca2+/CaM Signaling to hVps34. Cell Metab. 2008. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, M.G.; Hamilton, D.L.; Murray, J.T.; Taylor, P.M.; Baar, K. mVps34 is activated following high-resistance contractions. J. Physiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, M.G.; Hamilton, D.L.; Murray, J.T.; Baar, K. mVps34 is activated by an acute bout of resistance exercise. Biochem. Soc. Trans. 2007. [Google Scholar] [CrossRef] [PubMed]
- Mercan, F.; Lee, H.; Kolli, S.; Bennett, A.M. Novel Role for SHP-2 in Nutrient-Responsive Control of S6 Kinase 1 Signaling. Mol. Cell. Biol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002. [Google Scholar] [CrossRef] [PubMed]
- von Kügelgen, I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006, 110, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Moschella, M.C.; Watras, J.; Jayaraman, T.; Marks, A.R. Inositol 1,4,5-trisphosphate receptor in skeletal muscle: Differential expression in myofibers. J. Muscle. Res. Cell. Motil. 1995, 16, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kobzik, L.; Reid, M.B.; Bredt, D.S.; Stamler, J.S. Nitric oxide in skeletal muscle. Nature 1994, 372, 546. [Google Scholar] [CrossRef] [PubMed]
- White, N.; Burnstock, G. P2 receptors and cancer. Trends Pharmacol. Sci. 2006, 27, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kii, I.; Shimizu, N.; Tanaka, H.; Takeda, S. Direct reprogramming of fibroblasts into skeletal muscle progenitor cells by transcription factors enriched in undifferentiated subpopulation of satellite cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An expanded palette of genetically encoded Ca2+ indicators. Science 2011. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Koshino, Y.; Shibata, F.; Oki, T.; Nakajima, H.; Nosaka, T.; Kumagai, H. Retrovirus-mediated gene transfer and expression cloning: Powerful tools in functional genomics. Exp. Hematol. 2003, 31, 1007–1014. [Google Scholar] [CrossRef]
- Morita, S.; Kojima, T.; Kitamura, T. Plat-E: An efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Shimizu, N.; Tanaka, H.; Takeda, S. Enhancement of Satellite Cell Transplantation Efficiency by Leukemia Inhibitory Factor. J. Neuromuscul. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hyzewicz, J.; Tanihata, J.; Kuraoka, M.; Ito, N.; Miyagoe-Suzuki, Y.; Takeda, S. Low intensity training of mdx mice reduces carbonylation and increases expression levels of proteins involved in energy metabolism and muscle contraction. Free Radic. Biol. Med. 2015, 82, 122–136. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, N.; Ruegg, U.T.; Takeda, S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int. J. Mol. Sci. 2018, 19, 2804. https://doi.org/10.3390/ijms19092804
Ito N, Ruegg UT, Takeda S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. International Journal of Molecular Sciences. 2018; 19(9):2804. https://doi.org/10.3390/ijms19092804
Chicago/Turabian StyleIto, Naoki, Urs T. Ruegg, and Shin’ichi Takeda. 2018. "ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy" International Journal of Molecular Sciences 19, no. 9: 2804. https://doi.org/10.3390/ijms19092804




