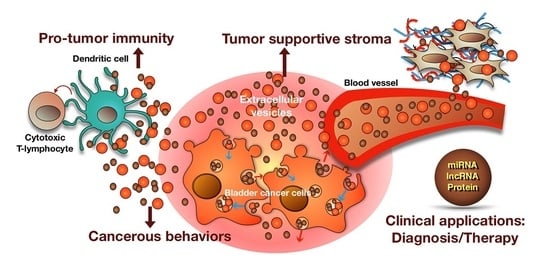
Extracellular Vesicles in Bladder Cancer: Biomarkers and Beyond
Abstract
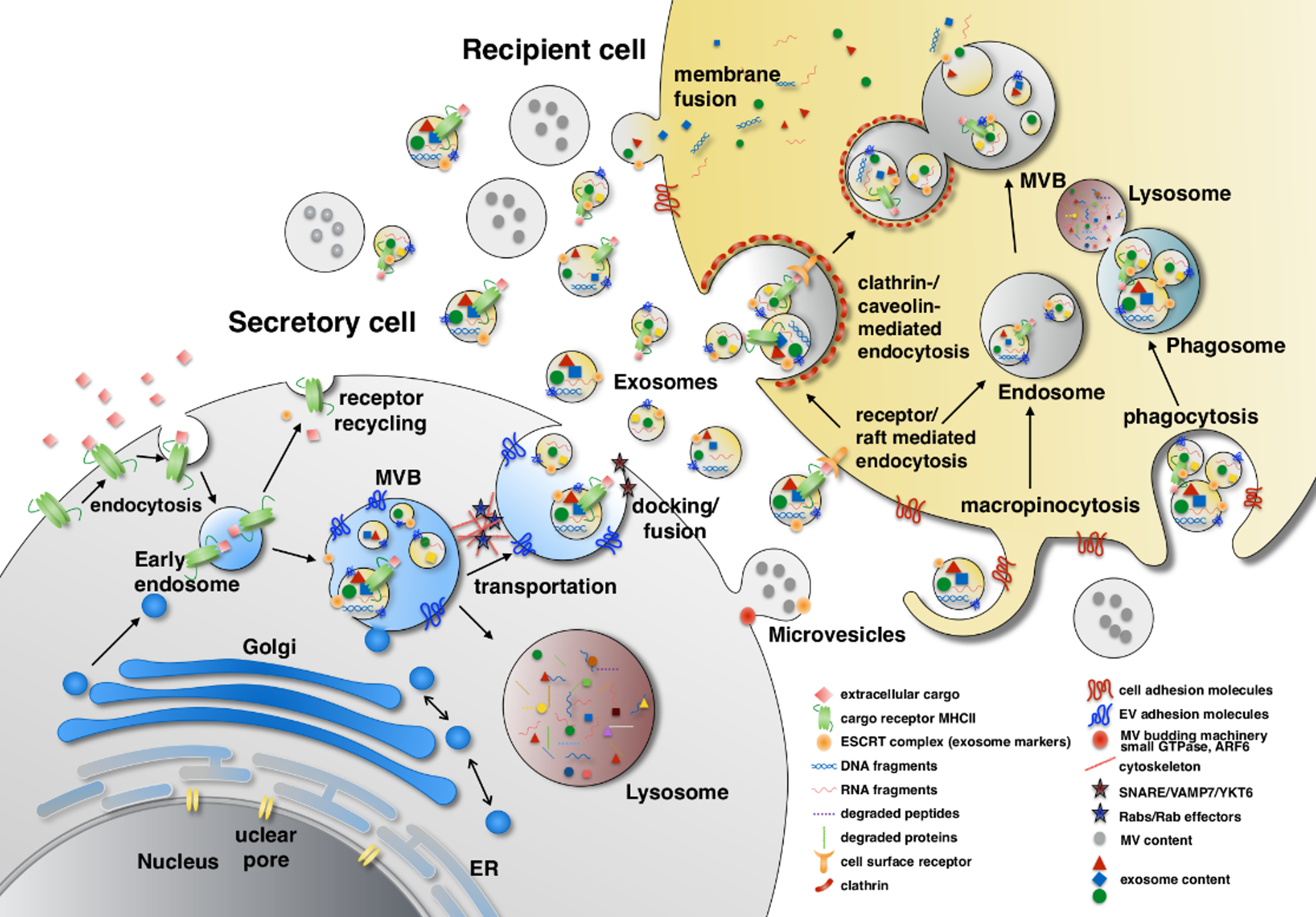
:1. Introduction
2. Oncogenic Properties of BCEVs
2.1. BCEVs in Neoplastic Transformation
2.2. BCEVs Promote Cancer Cell Progression by Mediating Communication between Tumor Cells
2.2.1. Proliferation
2.2.2. Migration and Invasion
2.3. BCEVs Promote Cancer Cell Progression by Mediating Tumor-Stroma Communication
3. Regulation of Immune Responses by BCEVs
3.1. Immune System Activation by BCEVs
3.2. Immune System Suppression by BCEVs
3.3. BCEVs in Promoting Inflammation
4. Therapeutic Application of BCEVs
4.1. EV-Mediated Delivery of Therapeutic Agents in BC
4.2. Prognosis and Diagnosis of BC Using EVs
5. Current Challenges and Future Prospects
5.1. Current Challenges
5.2. Future Prospects
6. Conclusions
Funding
Conflicts of Interest
References
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellon, D.M.; Davidson, S.M. Exosomes: Nanoparticles involved in cardioprotection? Circ. Res. 2014, 114, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Aalberts, M.; Stout, T.A.; Stoorvogel, W. Prostasomes: Extracellular vesicles from the prostate. Reproduction 2014, 147, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.; Lee, T.H.; Spinelli, C.; Chennakrishnaiah, S.; D’Asti, E.; Rak, J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin. Cell Dev. Biol. 2017, 67, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Urciuoli, E.; Giorda, E.; Scarsella, M.; Petrini, S.; Peruzzi, B. Osteosarcoma-derived extracellular vesicles induce a tumor-like phenotype in normal recipient cells. J. Cell. Physiol. 2018, 233, 6158–6172. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, K.; Cross-Knorr, S.; Dillard, C.; Pantazatos, D.; Del Tatto, M.; Mills, D.; Goldstein, L.; Renzulli, J.; Quesenberry, P.; Chatterjee, D. Reversal of chemosensitivity and induction of cell malignancy of a non-malignant prostate cancer cell line upon extracellular vesicle exposure. Mol. Cancer 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E.; Karras, J.G.; Murphy, T.F.; Barton, A.; Huang, H.F.S. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: Direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol. Cancer Ther. 2004, 3, 11–20. [Google Scholar] [PubMed]
- Bromberg, J. Stat proteins and oncogenesis. J. Clin. Investig. 2002, 109, 1139–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet, C.R.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Poulit, F. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFβ signaling. Mol. Cancer Res. 2018. [Google Scholar] [CrossRef]
- Mateescu, B.; Kowal, E.J.; van Balkom, B.W.; Bartel, S.; Bhattacharyya, S.N.; Buzas, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, X.H.; Wang, D.; Luo, C.L.; Chen, L.X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol. Med. Rep. 2013, 8, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Chen, W.; Xiang, A.; Wang, R.Q.; Chen, H.; Pan, J.J.; Pang, H.; An, H.L.; Wang, X.; Hou, H.L.; et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol. Cancer 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Beckham, C.J.; Olsen, J.; Yin, P.N.; Wu, C.H.; Ting, H.J.; Hagen, F.K.; Scosyrev, E.; Messing, E.M.; Lee, Y.F. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J. Urol. 2014, 192, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Silvers, C.R.; Liu, Y.R.; Wu, C.H.; Miyamoto, H.; Messing, E.M.; Lee, Y.F. Identification of extracellular vesicle-borne periostin as a feature of muscle-invasive bladder cancer. Oncotarget 2016, 7, 23335–23345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzen, C.; Greco, K.; Blackwell, R.; Foreman, K.; Gupta, G. Urothelial Cells Undergo Epithelial to Mesenchymal Transition after Exposure to Muscle Invasive Bladder Cancer Exosomes. J. Urol. 2015, 193, E605–E606. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Jeppesen, D.K.; Laurberg, J.R.; Boysen, A.T.; Bramsen, J.B.; Primdal-Bengtson, B.; Hendrix, A.; Lamy, P.; Dagnaes-Hansen, F.; Rasmussen, M.H.; et al. Cellular Disposal of miR23b by RAB27-Dependent Exosome Release Is Linked to Acquisition of Metastatic Properties. Cancer Res. 2014, 74, 5758–5771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.Y.; Zhang, C.L.; Lum, D.; Druso, J.E.; Blank, B.; Wilson, K.F.; Welm, A.; Antonyak, M.A.; Cerione, R.A. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Chavakis, E.; Czabanka, M.A.; Langer, H.F.; Fraemohs, L.; Economopoulou, M.; Kundu, R.K.; Orlandi, A.; Zheng, Y.Y.; Prieto, D.A.; et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 2008, 322, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Lima, L.G.; Moller, A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Semin. Cell Dev. Biol. 2017, 67, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.R.; Lee, Y.F. Bladder cancer extracellular vesicle facilitate metastasis. Unpublished; manuscript in preparation.
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, J.N.; Akbani, R.; Broom, B.M.; Wang, W.Y.; Verhaak, R.G.W.; McConkey, D.; Lerner, S.; Morgan, M.; Creighton, C.J.; Smith, C.; et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Sjodahl, G.; Lauss, M.; Lovgren, K.; Chebil, G.; Gudjonsson, S.; Veerla, S.; Patschan, O.; Aine, M.; Ferno, M.; Ringner, M.; et al. A Molecular Taxonomy for Urothelial Carcinoma. Clin. Cancer Res. 2012, 18, 3377–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Zuo, B.F.; Lu, Z.; Gao, X.J.; You, A.B.; Wu, C.X.; Du, Z.; Yin, H.F. Tumor-Derived Exosomes Elicit Tumor Suppression in Murine Hepatocellular Carcinoma Models and Humans In Vitro. Hepatology 2016, 64, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.; Wu, H.Q.; Sun, B.Z.; Zhang, G.L.; Zhan, S.Q.; Zhang, R.; Zhou, L. Exosome-loaded dendritic cells elicit tumor-specific CD8(+) cytotoxic T cells in patients with glioma. J. Neurooncol. 2011, 104, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Wu, X.H.; Zhang, Y.; Xia, Y.G.; Luo, C.L. Exosomes derived form bladder transitional cell carcinoma cells induce CTL cytotoxicity in vitro. Zhonghua Zhong Liu Za Zhi 2009, 31, 738–741. [Google Scholar] [PubMed]
- Ljunggren, H.G.; Karre, K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Whiteside, T.L. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem. Soc. Trans. 2013, 41, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, H.; Kuranaga, Y.; Kumazaki, M.; Sugito, N.; Yoshikawa, Y.; Takai, T.; Taniguchi, K.; Ito, Y.; Akao, Y. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. J. Immunol. 2017, 199, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Salimu, J.; Webber, J.; Gurney, M.; Al-Taei, S.; Clayton, A.; Tabi, Z. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J. Extracell. Vesicles 2017, 6, 1368823. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, R.; Li, S.; Luo, R. Immunosuppression of breast cancer cells mediated by transforming growth factor-beta in exosomes from cancer cells. Oncol. Lett. 2016, 11, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; McKinney, K.Q.; Pavlopoulos, A.J.; Niu, M.; Kang, J.W.; Oh, J.W.; Kim, K.P.; Hwang, S. Altered Proteome of Extracellular Vesicles Derived from Bladder Cancer Patients Urine. Mol. Cells 2018, 41, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sutoh, M.; Hatakeyama, S.; Mori, K.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; Habuchi, T.; et al. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int. J. Oncol. 2012, 40, 1831–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, T.O.; Borre, M.; Nexo, E.; Sorensen, B.S. Co-expression of HER3 and MUC1 is associated with a favourable prognosis in patients with bladder cancer. BJU Int. 2015, 115, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Bramswig, K.H.; Poettler, M.; Unseld, M.; Wrba, F.; Uhrin, P.; Zimmermann, W.; Zielinski, C.C.; Prager, G.W. Soluble carcinoembryonic antigen activates endothelial cells and tumor angiogenesis. Cancer Res. 2013, 73, 6584–6596. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.; Markel, G.; Arnon, T.I.; Gruda, R.; Wong, H.; Gray-Owen, S.D.; Mandelboim, O. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J. Immunol. 2005, 174, 6692–6701. [Google Scholar] [CrossRef] [PubMed]
- Adada, M.M.; Canals, D.; Jeong, N.; Kelkar, A.D.; Hernandez-Corbacho, M.; Pulkoski-Gross, M.J.; Donaldson, J.C.; Hannun, Y.A.; Obeid, L.M. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 2015, 29, 4654–4669. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Phang, J.M.; Yu, J.; Harrop, S.J.; Sokolova, A.V.; Duff, A.P.; Wilk, K.E.; Alkhamici, H.; Breit, S.N.; Valenzuela, S.M.; et al. CLIC proteins, ezrin, radixin, moesin and the coupling of membranes to the actin cytoskeleton: A smoking gun? Biochim. Biophys. Acta 2014, 1838, 643–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, J.; Liu, S.; Xu, Y.; Wang, C.; Lin, Z.; Qin, Y.; Liu, S. Ezrin protein overexpression predicts the poor prognosis of pancreatic ductal adenocarcinomas. Exp. Mol. Pathol. 2015, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yago, T.; Zhang, N.; Abdisalaam, S.; Alexandrakis, G.; Rodgers, W.; McEver, R.P. Cytoskeletal regulation of CD44 membrane organization and interactions with E-selectin. J. Biol. Chem. 2014, 289, 35159–35171. [Google Scholar] [CrossRef] [PubMed]
- Silvers, C.R.; Miyamoto, H.; Messing, E.M.; Netto, G.J.; Lee, Y.F. Characterization of urinary extracellular vesicle proteins in muscle-invasive bladder cancer. Oncotarget 2017, 8, 91199–91208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perl, A.; Hanczko, R.; Telarico, T.; Oaks, Z.; Landas, S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 2011, 17, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, Z.; Otta Oshiro, R.; Redruello, A.; Lopez-Martin, S.; Gutierrez-Vazquez, C.; Morato, E.; Marina, A.I.; Olivier Gomez, C.; Yanez-Mo, M. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur. J. Pharm. Sci. 2017, 98, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Bergam, P.; Di Cicco, A.; Hurbain, I.; Lo Cicero, A.; Dingli, F.; Palmulli, R.; Fort, C.; Potier, M.C.; Schurgers, L.J.; et al. Apolipoprotein E Regulates Amyloid Formation within Endosomes of Pigment Cells. Cell Rep. 2015, 13, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodar, B.W.; Kittel, A.; Paloczi, K.; Vukman, K.V.; Osteikoetxea, X.; Szabo-Taylor, K.; Nemeth, A.; Sperlagh, B.; Baranyai, T.; Giricz, Z.; et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep. 2016, 6, 24316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avraham-Davidi, I.; Ely, Y.; Pham, V.N.; Castranova, D.; Grunspan, M.; Malkinson, G.; Gibbs-Bar, L.; Mayseless, O.; Allmog, G.; Lo, B.; et al. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat. Med. 2012, 18, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, L.N.; Ponnusamy, T.; Philip, S.; Mukhopadhyay, R.; Kakkar, V.V.; Mundkur, L. Hypercholesterolemia Induced Immune Response and Inflammation on Progression of Atherosclerosis in Apob(tm2Sgy) Ldlr(tm1Her)/J Mice. Lipids 2015, 50, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Bergin, C.; O’Leary, A.; McCreary, C.; Sabra, K.; Mulcahy, F. Treatment of Kaposi’s sarcoma with liposomal doxorubicin. Am. J. Health Syst. Pharm. 1995, 52, 2001–2004. [Google Scholar] [PubMed]
- Sun, D.M.; Zhuang, X.Y.; Zhang, S.Q.; Deng, Z.B.; Grizzle, W.; Miller, D.; Zhang, H.G. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv. Drug Deliv. Rev. 2013, 65, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.Y.; Xiang, X.Y.; Grizzle, W.; Sun, D.M.; Zhang, S.Q.; Axtell, R.C.; Ju, S.W.; Mu, J.Y.; Zhang, L.F.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Franzen, C.A.; Simms, P.E.; Van Huis, A.F.; Foreman, K.E.; Kuo, P.C.; Gupta, G.N. Characterization of Uptake and Internalization of Exosomes by Bladder Cancer Cells. Biomed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. PLK-1 Silencing in Bladder Cancer by siRNA Delivered With Exosomes. Urology 2016, 91. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ma, J.W.; Liang, X.Y.; Tang, K.; Liu, Y.Y.; Yin, X.A.; Zhang, Y.; Zhang, H.F.; Xu, P.W.; Chen, D.G.; et al. Pre-instillation of tumor microparticles enhances intravesical chemotherapy of nonmuscle-invasive bladder cancer through a lysosomal pathway. Biomaterials 2017, 113, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Bonilla, C.J.; Lee, Y.F. BCG internalization releases increased levels of immune-active extracellular vesicles. Unpublished; manuscript in preparation.
- Zhou, H.; Cheruvanky, A.; Hu, X.; Matsumoto, T.; Hiramatsu, N.; Cho, M.E.; Berger, A.; Leelahavanichkul, A.; Doi, K.; Chawla, L.S.; et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008, 74, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, P.G.; You, S.; Lee, J.E.; Hwang, D.; Baek, M.C. Urinary exosomes and proteomics. Mass Spectrom. Rev. 2011, 30, 1185–1202. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Kumar, C.; Zhang, Y.; Olsen, J.V.; Mann, M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006, 7, R80. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.G.; Lee, J.E.; You, S.; Kim, T.K.; Cho, J.H.; Kim, I.S.; Kwon, T.H.; Kim, C.D.; Park, S.H.; Hwang, D.; et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 2011, 11, 2459–2475. [Google Scholar] [CrossRef] [PubMed]
- Knepper, M.A. Common sense approaches to urinary biomarker study design. J. Am. Soc. Nephrol. 2009, 20, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.; Pisitkun, T.; Knepper, M.A. Urinary exosomes: Is there a future? Nephrol. Dial. Transplant. 2008, 23, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.D.; Abdel-Bakky, M.S.; Gutwein, P.; Altevogt, P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Manganelli, L.; Woollard, J.R.; Masyuk, A.I.; Masyuk, T.V.; Tammachote, R.; Huang, B.Q.; Leontovich, A.A.; Beito, T.G.; Madden, B.J.; et al. Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 2009, 20, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Qiu, F.; Qiu, Z. Comprehensive analysis of low-abundance proteins in human urinary exosomes using peptide ligand library technology, peptide OFFGEL fractionation and nanoHPLC-chip-MS/MS. Electrophoresis 2010, 31, 3797–3807. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Camussi, G.; Valadi, H.; Nazarenko, I.; Ekstrom, K.; Wang, X.; Principe, S.; Shah, N.; Ashraf, N.M.; Fatima, F.; et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 2014, 11, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hill, S.; Luther, J.M.; Hachey, D.L.; Schey, K.L. Proteomic analysis of urine exosomes by multidimensional protein identification technology (MudPIT). Proteomics 2012, 12, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Lai, Y.F.; Tang, P.; Chien, K.Y.; Yu, J.S.; Tsai, C.H.; Chen, H.W.; Wu, C.C.; Chung, T.; Hsu, C.W.; et al. Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients. J. Proteome Res. 2012, 11, 5611–5629. [Google Scholar] [CrossRef] [PubMed]
- Principe, S.; Jones, E.E.; Kim, Y.; Sinha, A.; Nyalwidhe, J.O.; Brooks, J.; Semmes, O.J.; Troyer, D.A.; Lance, R.S.; Kislinger, T.; et al. In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine. Proteomics 2013, 13, 1667–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Principe, S.; Kim, Y.; Fontana, S.; Ignatchenko, V.; Nyalwidhe, J.O.; Lance, R.S.; Troyer, D.A.; Alessandro, R.; Semmes, O.J.; Kislinger, T.; et al. Identification of prostate-enriched proteins by in-depth proteomic analyses of expressed prostatic secretions in urine. J. Proteome Res. 2012, 11, 2386–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiblich, A. Recent Developments in the Search for Urinary Biomarkers in Bladder Cancer. Curr. Urol. Rep. 2017, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.; Kogej, M.; Fechner, G.; Von Ruecker, A.; Bastian, P.J.; Von Pezold, J.; Vorreuther, R.; Lummen, G.; Muller, S.C.; Ellinger, J. Cell-free serum DNA in patients with bladder cancer: Results of a prospective multicenter study. Anticancer Res. 2012, 32, 3119–3124. [Google Scholar] [PubMed]
- Casadio, V.; Calistri, D.; Tebaldi, M.; Bravaccini, S.; Gunelli, R.; Martorana, G.; Bertaccini, A.; Serra, L.; Scarpi, E.; Amadori, D.; et al. Urine cell-free DNA integrity as a marker for early bladder cancer diagnosis: Preliminary data. Urol. Oncol. 2013, 31, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, J. Clinical applications of urinary cell-free DNA in cancer: Current insights and promising future. Am. J. Cancer Res. 2017, 7, 2318–2332. [Google Scholar] [PubMed]
- Berrondo, C.; Flax, J.; Kucherov, V.; Siebert, A.; Osinski, T.; Rosenberg, A.; Fucile, C.; Richheimer, S.; Beckham, C.J. Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS ONE 2016, 11, e0147236. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.G.; Kong, M.Q.; Zhou, S.; Sheng, Y.F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.J.; Demirci, U.; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017, 7, 46224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juracek, J.; Peltanova, B.; Dolezel, J.; Fedorko, M.; Pacik, D.; Radova, L.; Vesela, P.; Svoboda, M.; Slaby, O.; Stanik, M. Genome-wide identification of urinary cell-free microRNAs for non-invasive detection of bladder cancer. J. Cell. Mol. Med. 2018, 22, 2033–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.Y.; Chang, C.H.; Wu, H.C.; Lin, C.C.; Chang, K.P.; Yang, C.R.; Huang, C.P.; Hsu, W.H.; Chang, C.T.; Chen, C.J. Proteome Profiling of Urinary Exosomes Identifies Alpha 1-Antitrypsin and H2B1K as Diagnostic and Prognostic Biomarkers for Urothelial Carcinoma. Sci. Rep. 2016, 6, 34446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.D.; Sullivan, T.B.; Humphrey, J.; Logvinenko, T.; Summerhayes, K.A.; Kozinn, S.; Harty, N.; Summerhayes, I.C.; Libertino, J.A.; Holway, A.H.; et al. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am. J. Transl. Res. 2015, 7, 2500–2509. [Google Scholar] [PubMed]
- Armstrong, D.A.; Green, B.B.; Seigne, J.D.; Schned, A.R.; Marsit, C.J. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol. Cancer 2015, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Fujita, K.; Jingushi, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Ueda, Y.; Tanigawa, G.; Yoshioka, I.; Ueda, K.; et al. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget 2017, 8, 24668–24678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardini, B.; Cordero, F.; Naccarati, A.; Viberti, C.; Birolo, G.; Oderda, M.; Di Gaetano, C.; Arigoni, M.; Martina, F.; Calogero, R.A.; et al. microRNA profiles in urine by next-generation sequencing can stratify bladder cancer subtypes. Oncotarget 2018, 9, 20658–20669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heba Fanous, T.S. Kimberly Rieger-Christ. Distinct exosomalL miRNA profiles in chemoresistant bladder carcinoma cell lines. J. Urol. 2017, 197, 2. [Google Scholar]
- Yasui, T.; Yanagida, T.; Ito, S.; Konakade, Y.; Takeshita, D.; Naganawa, T.; Nagashima, K.; Shimada, T.; Kaji, N.; Nakamura, Y.; et al. Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci. Adv. 2017, 3, e1701133. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, S.; Holters, S.; Ohlmann, C.H.; Bohle, R.; Stockle, M.; Ostenfeld, M.S.; Dyrskjot, L.; Junker, K.; Heinzelmann, J. Exosomes of invasive urothelial carcinoma cells are characterized by a specific miRNA expression signature. Oncotarget 2017, 8, 58278–58291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.Z.; Lau, K.M.; Chan, E.S.; Wang, G.; Szeto, C.C.; Wong, K.; Choy, R.K.; Ng, C.F. Cell-free urinary microRNA-99a and microRNA-125b are diagnostic markers for the non-invasive screening of bladder cancer. PLoS ONE 2014, 9, e100793. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, H.W.; Kim, W.T.; Kim, Y.J.; Yun, S.J.; Lee, S.C.; Kim, W.J. Cell-Free microRNA-214 From Urine as a Biomarker for Non-Muscle-Invasive Bladder Cancer. Korean J. Urol. 2013, 54, 791–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley, D.M.; Sheman, N.E.; Nelson, K.; Theodorescu, D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J. Proteome Res. 2008, 7, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Fontana, S.; Saieva, L.; Taverna, S.; Alessandro, R. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: State of the art and new perspectives. Proteomics 2013, 13, 1581–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welton, J.L.; Khanna, S.; Giles, P.J.; Brennan, P.; Brewis, I.A.; Staffurth, J.; Mason, M.D.; Clayton, A. Proteomics analysis of bladder cancer exosomes. Mol. Cell. Proteom. 2010, 9, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Saxena, S.; Agrawal, U. Exosomal protein interactors as emerging therapeutic targets in urothelial bladder cancer. J. Egypt. Natl. Cancer Inst. 2015, 27, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.L.; Khosroheidari, M.; Ravi, R.K.; DiStefano, J.K. Comparison of protein, microRNA and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012, 82, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Musante, L.; Saraswat, M.; Duriez, E.; Byrne, B.; Ravida, A.; Domon, B.; Holthofer, H. Biochemical and physical characterisation of urinary nanovesicles following CHAPS treatment. PLoS ONE 2012, 7, e37279. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Liga, A.; Vliegenthart, A.D.B.; Oosthuyzen, W.; Dear, J.W.; Kersaudy-Kerhoas, M. Exosome isolation: A microfluidic road-map. Lab Chip 2015, 15, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, S.M.; Antonyak, M.A.; Cerione, R.A.; Kirby, B.J. Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomed. Microdevices 2014, 16, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Llama, P.; Khositseth, S.; Gonzales, P.A.; Star, R.A.; Pisitkun, T.; Knepper, M.A. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int. 2010, 77, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witwer, K.W.; Buzas, E.I.; Bemis, L.T.; Bora, A.; Lasser, C.; Lotvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotvall, J.; Rajendran, L.; Gho, Y.S.; Thery, C.; Wauben, M.; Raposo, G.; Sjostrand, M.; Taylor, D.; Telemo, E.; Breakefield, X.O. The launch of Journal of Extracellular Vesicles (JEV), the official journal of the International Society for Extracellular Vesicles—About microvesicles, exosomes, ectosomes and other extracellular vesicles. J. Extracell. Vesicles 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Sabbisetti, V.S.; Bonventre, J.V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010, 78, 486–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.W.A.; Toh, Q.C.; Teo, B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research—When and why. Singapore Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Mattix, H.J.; Hsu, C.Y.; Shaykevich, S.; Curhan, G. Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J. Am. Soc. Nephrol. 2002, 13, 1034–1039. [Google Scholar] [PubMed]
- Mitch, W.E.; Collier, V.U.; Walser, M. Creatinine Metabolism in Chronic Renal-Failure. Clin. Res. 1978, 26, A636. [Google Scholar] [CrossRef]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Prunotto, M.; Farina, A.; Lane, L.; Pernin, A.; Schifferli, J.; Hochstrasser, D.F.; Lescuyer, P.; Moll, S. Proteomic analysis of podocyte exosome-enriched fraction from normal human urine. J. Proteom. 2013, 82, 193–229. [Google Scholar] [CrossRef] [PubMed]
- Lentz, M.R. Continuous whole blood UltraPheresis procedure in patients with metastatic cancer. J. Biol. Response Mod. 1989, 8, 511–527. [Google Scholar] [PubMed]
- Tullis, R.H.; Duffin, R.P.; Handley, H.H.; Sodhi, P.; Menon, J.; Joyce, J.A.; Kher, V. Reduction of hepatitis C virus using lectin affinity plasmapheresis in dialysis patients. Blood Purif. 2009, 27, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Marleau, A.M.; Chen, C.S.; Joyce, J.A.; Tullis, R.H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 2012, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wang, X.; Zhang, J.; Lai, R. MicroRNA-599 suppresses glioma progression by targeting RAB27B. Oncol. Lett. 2018, 16, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xitong, D.; Xiaorong, Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene 2016, 575, 377–384. [Google Scholar] [CrossRef] [PubMed]

| miRNA | Regulation | Sample Sources | Reference |
|---|---|---|---|
| miR-21 | up | urine & BC cells lines | [85,86,87,88,89] |
| miR-200c | up | urine | [85,86,88] |
| miR-23b | up | urine | [19,90] |
| miR-513b-5p | up | urine | [90,91] |
| miR-183 | up | urine | [88,92] |
| miR-205 | up | urine from NMIBC patients | [86,88] |
| miR-16-1-3p, miR-28-5p, miR-92a-2-5p, miR-142-3p, miR-195-3p, miR-196b-5p, miR-299-3p, miR-492, miR-601, miR-619-5p, miR-3155a, miR-3162-5p, miR-3678-3p, miR-4283, miR-4295, miR-4311, miR-4531, miR-5096, miR-5187-5p | up | urine | [90] |
| miR-155-5p, miR-132-3p, miR-31-5p, miR-15a-5p | up | urine | [87] |
| miR-93, miR-940 | up | urine | [85] |
| miR-16, miR-96 | up | urine | [92] |
| miR-486-5p, miR-205-5p, let-7i-5p | up | urine from NMIBC/(G1 + G2) | [88] |
| miR-106b-3p, let-7c-5p, miR-486-5p, miR-151a-3p, miR-200c-3p, miR-183-5p, miR-185-5p, miR-224-5p | up | urine from NMIBC/G3 | |
| miR-4454, miR-720/3007a, miR-29-3p | up | urine from NMIBC | [86] |
| miR-214 | up | urine from NMIBC | [93] |
| miR-503-5p, miR-145-5p, miR-3158-3p, miR-30a-3p | up | urine from MIBC | [91] |
| miR-106b-3p, miR-486-5p, miR-205-5p, miR-451a, miR-25-3p, miR-7-1-5p, miR-146a-5p | up | urine from MIBC | [88] |
| miR-1, miR-99a, miR-125b, miR-133b, miR-143, miR-1207-5p | down | urine | [92] |
| let-7f-2-3p, miR-520c-3p, miR-4783-5p | down | urine | [90] |
| miR-30c-2-5p, miR-30a-5p | down | urine from NMIBC/(G1 + G2) | [88] |
| miR-30a-5p, miR-30c-2-5p, miR-10b-5p | down | urine from NMIBC/G3 | |
| miR-30a-5p, let-7c-5p | down | urine from MIBC | |
| miR-27b-3p | down | BC cells | [91] |
| miR-let-7i-3p | down | BC cells | [89] |
| miR-29c-5p, miR-146b-5p, miR-200a-3p, miR-200b-3p, miR-141-3p | down | BC cells | [91] |
| Protein ID | Sample Sources | Validated | Proteomic Detection |
|---|---|---|---|
| EHD4 | urine and BC cells | [47] | [16,38,94] |
| HEXB | urine and BC cells | [16,38] | |
| ANXA; SND1 | urine and BC cells | [16,95] | |
| S100A4 | urine and BC cells | [16] | |
| TALDO1 | urine and BC cells | [16] | |
| MUC1 | urine and BC cells | [38,96] | [95] |
| EPS8 | urine | [38] | [94] |
| CEAM5 | urine | ||
| CD44; BSG | BC cells | [96] | |
| ITGB1; ITGA6; CD36; CD73; CD10; CD147; 5T4 | BC cells | ||
| NRAS; MUC4 | urine | [94] | |
| SERPINA1 H2B1K | urine | [84] | |
| TACSTD2 | urine | [74] | |
| EDIL3 | urine and BC cells | [16] | |
| POSTN | urine and BC cells | [17] | |
| CTNNB1; CDC42 | urine and BC cells | [95,97] | |
| 14-3-3; ALIX; B2M; EGFR; EZR; FSCN1; LGALS; GST; MSN; PRDX1; PTGFRN; RDX; TAGLN2 | BC cells | [95] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-R.; Ortiz-Bonilla, C.J.; Lee, Y.-F. Extracellular Vesicles in Bladder Cancer: Biomarkers and Beyond. Int. J. Mol. Sci. 2018, 19, 2822. https://doi.org/10.3390/ijms19092822
Liu Y-R, Ortiz-Bonilla CJ, Lee Y-F. Extracellular Vesicles in Bladder Cancer: Biomarkers and Beyond. International Journal of Molecular Sciences. 2018; 19(9):2822. https://doi.org/10.3390/ijms19092822
Chicago/Turabian StyleLiu, Yu-Ru, Carlos J. Ortiz-Bonilla, and Yi-Fen Lee. 2018. "Extracellular Vesicles in Bladder Cancer: Biomarkers and Beyond" International Journal of Molecular Sciences 19, no. 9: 2822. https://doi.org/10.3390/ijms19092822