Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer
Abstract
:1. Introduction
2. Metabolic Background of Cancer Cachexia and Sarcopenia
2.1. Adipose Tissue Depletion in Cancer Cachexia
2.2. Skeletal Muscle Depletion in Cancer Cachexia
3. Evaluation of Sarcopenia Using Computed Tomography (CT) Images
3.1. Measurement of Skeletal Muscle Mass Using CT Images
3.2. Skeletal Muscle Index (SMI)
3.3. Psoas Muscle Index (PMI)
3.4. Skeletal Muscle Density
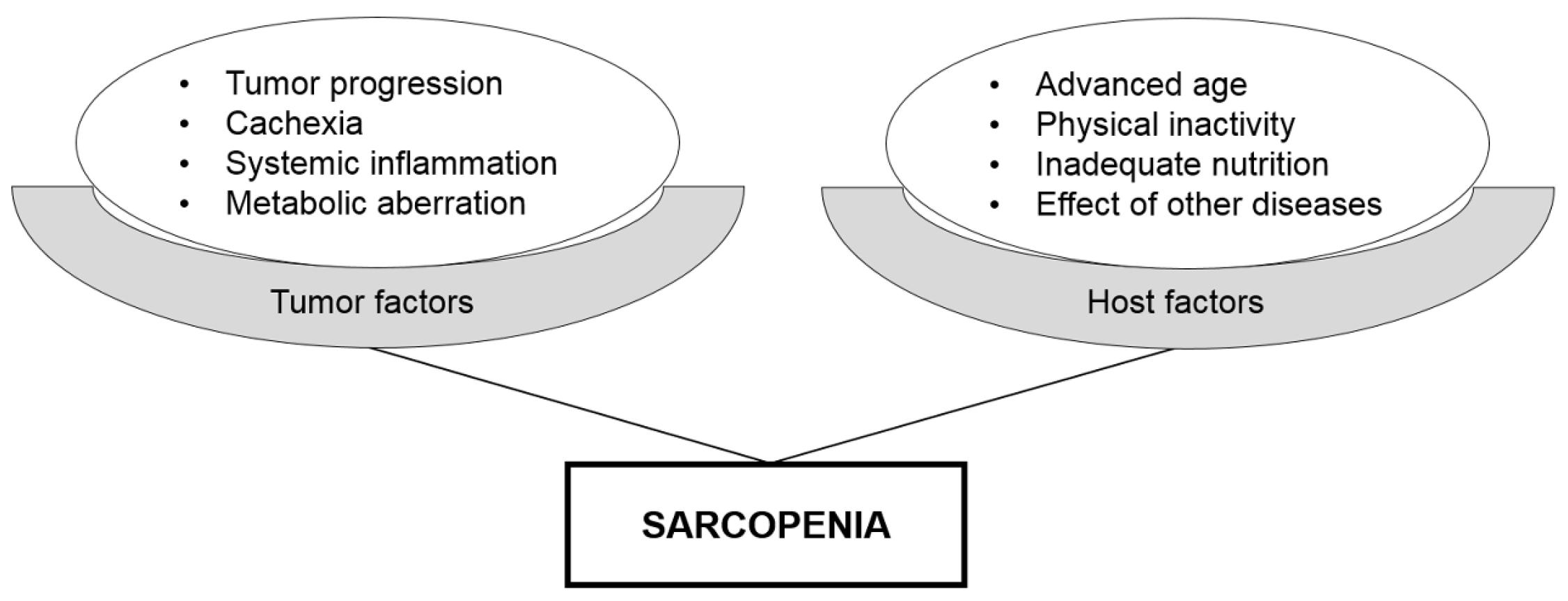
4. Hybrid Nature of Sarcopenia as a Prognostic Biomarker
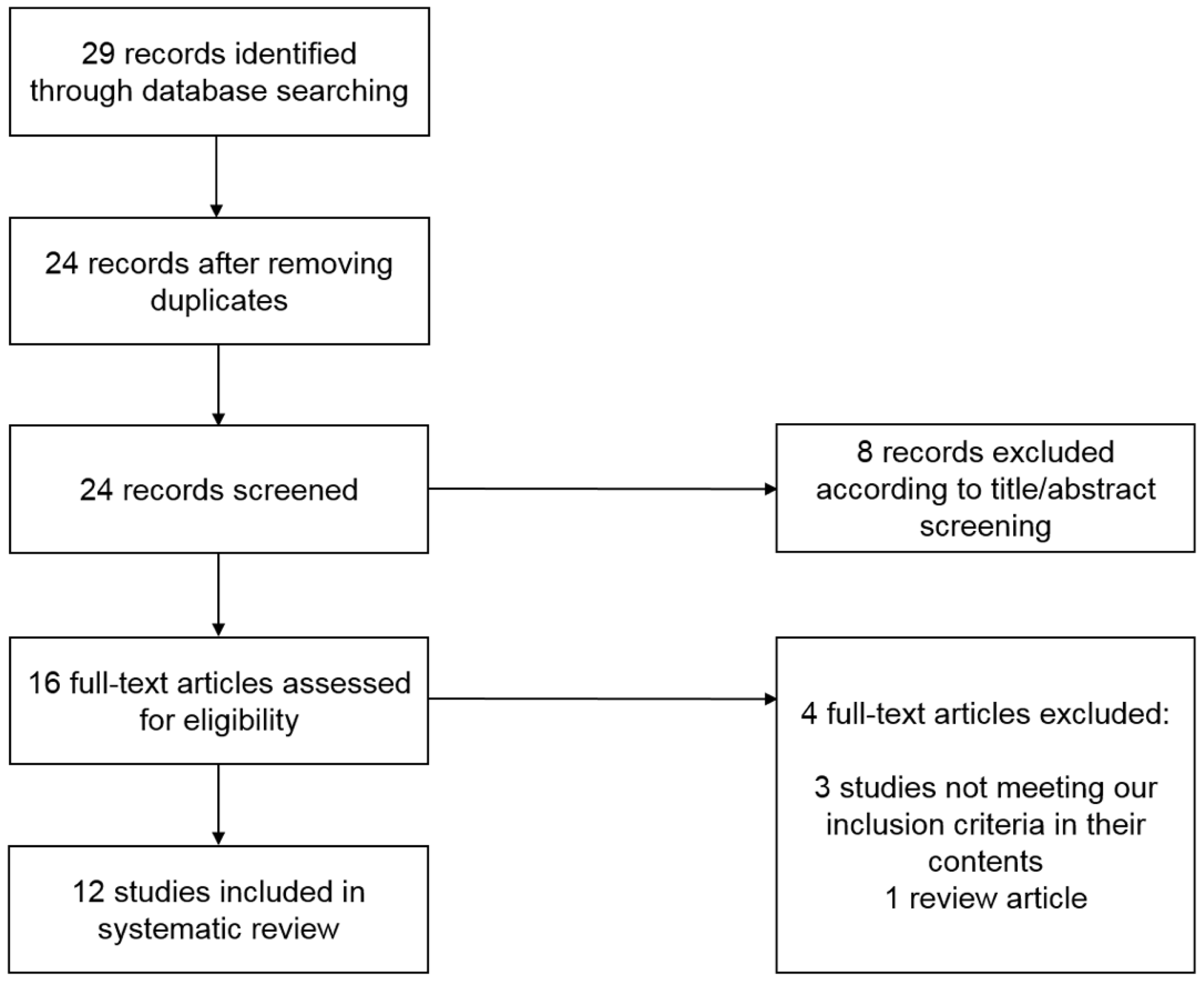
5. Systematic Literature Review
6. Prognostic Role of Sarcopenia in Bladder Cancer
6.1. Survival after a Radical Cystectomy
6.2. Survival in Inoperable Advanced Disease
7. Prognostic Role of Changes in Skeletal Muscle Mass in Bladder Cancer
8. Therapeutic Interventions for Sarcopenia
8.1. Nutritional Support
8.2. Medications
8.2.1. n-3 Fatty Acids
8.2.2. ActR2B Antagonist
8.2.3. Medications for Insulin Resistance
8.2.4. Inhibitors for Lipolysis and Fatty Acid Oxidation
8.2.5. Hormonal Replacement Therapy
8.3. Exercise
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Lebret, T.; Comperat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernandez, V.; Espinos, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Dalbagni, G.; Genega, E.; Hashibe, M.; Zhang, Z.F.; Russo, P.; Herr, H.; Reuter, V. Cystectomy for bladder cancer: A contemporary series. J. Urol. 2001, 165, 1111–1116. [Google Scholar] [CrossRef]
- Bellmunt, J.; von der Maase, H.; Mead, G.M.; Skoneczna, I.; De Santis, M.; Daugaard, G.; Boehle, A.; Chevreau, C.; Paz-Ares, L.; Laufman, L.R.; et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012, 30, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Sundahl, N.; Rottey, S.; De Maeseneer, D.; Ost, P. Pembrolizumab for the treatment of bladder cancer. Expert Rev. Anticancer Ther. 2018, 18, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Garatachea, N.; Lucia, A. Genes and the ageing muscle: A review on genetic association studies. Age 2013, 35, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexia Sarcopenia Muscle 2016, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cosqueric, G.; Sebag, A.; Ducolombier, C.; Thomas, C.; Piette, F.; Weill-Engerer, S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br. J. Nutr. 2006, 96, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, Y.P.; Shin, H.J.; Lee, W. Associations of Sarcopenia and Sarcopenic Obesity With Metabolic Syndrome Considering Both Muscle Mass and Muscle Strength. J. Prev. Med. Public Health 2016, 49, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, Y.H.; Huh, J.H.; Kang, D.R.; Rhee, Y.; Lim, S.K. Early-stage chronic kidney disease, insulin resistance, and osteoporosis as risk factors of sarcopenia in aged population: The fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008–2009. Osteoporos. Int. 2014, 25, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, J.J.; Park, Y.S. Relationship between sarcopenic obesity and cardiovascular disease risk as estimated by the Framingham risk score. J. Korean Med. Sci. 2015, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Ida, S.; Baba, Y.; Ishimoto, T.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ohuchi, M.; Nakamura, K.; Kiyozumi, Y.; et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis. Esophagus 2016, 29, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Go, S.I.; Park, M.J.; Song, H.N.; Kim, H.G.; Kang, M.H.; Lee, H.R.; Kim, Y.; Kim, R.B.; Lee, S.I.; Lee, G.W. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Cachexia Sarcopenia Muscle 2016, 7, 567–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabel, M.S.; Lee, J.; Cai, S.; Englesbe, M.J.; Holcombe, S.; Wang, S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann. Surg. Oncol. 2011, 18, 3579–3585. [Google Scholar] [CrossRef] [PubMed]
- Psutka, S.P.; Boorjian, S.A.; Moynagh, M.R.; Schmit, G.D.; Costello, B.A.; Thompson, R.H.; Stewart-Merrill, S.B.; Lohse, C.M.; Cheville, J.C.; Leibovich, B.C.; et al. Decreased Skeletal Muscle Mass is Associated with an Increased Risk of Mortality after Radical Nephrectomy for Localized Renal Cell Cancer. J. Urol. 2016, 195, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Nakanishi, Y.; Kataoka, M.; Tobisu, K.; Koga, F. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. J. Urol. 2016, 195, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, K.L.; Ke, J.Y.; Tian, M.; Cole, R.M.; Andridge, R.R.; Belury, M.A. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Boil. Ther. 2015, 16, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Eder, S.; Schauer, S.; Diwoky, C.; Temmel, H.; Guertl, B.; Gorkiewicz, G.; Tamilarasan, K.P.; Kumari, P.; Trauner, M.; et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011, 333, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Fukawa, T.; Yan-Jiang, B.C.; Min-Wen, J.C.; Jun-Hao, E.T.; Huang, D.; Qian, C.N.; Ong, P.; Li, Z.; Chen, S.; Mak, S.Y.; et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat. Med. 2016, 22, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.L.; Martignoni, M.E.; Bachmann, J.; Fechtner, K.; Friess, H.; Kinscherf, R.; Hildebrandt, W. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. 2007, 85, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Smith, J.D.; Chambers, M.A.; McLoughlin, T.J.; Reid, M.B. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am. J. Physiol. Cell Physiol. 2008, 295, C986–C993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julienne, C.M.; Dumas, J.F.; Goupille, C.; Pinault, M.; Berri, C.; Collin, A.; Tesseraud, S.; Couet, C.; Servais, S. Cancer cachexia is associated with a decrease in skeletal muscle mitochondrial oxidative capacities without alteration of ATP production efficiency. J. Cachexia Sarcopenia Muscle 2012, 3, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, N.A.; Skipworth, R.J.; Macdonald, A.J.; Greig, C.A.; Ross, J.A.; Fearon, K.C. Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, H.; Koga, F. Impact of sarcopenia in the management of urological cancer patients. Expert Rev. Anticancer Ther. 2017, 17, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulver, M.W.; Dohm, G.L. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc. Nutr. Soc. 2004, 63, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrentschuk, N.; Colombo, R.; Hakenberg, O.W.; Lerner, S.P.; Mansson, W.; Sagalowsky, A.; Wirth, M.P. Prevention and management of complications following radical cystectomy for bladder cancer. Eur. Urol. 2010, 57, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zhu, Y.; Gu, C.; Yao, X.; Shen, Y.; Dai, B.; Zhang, S.; Zhang, H.; Cheng, J.; Ye, D. Lower skeletal muscle index and early complications in patients undergoing radical cystectomy for bladder cancer. World J. Surg. Oncol. 2014, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.B.; Deal, A.M.; Yu, H.; Boyd, B.; Matthews, J.; Wallen, E.M.; Pruthi, R.S.; Woods, M.E.; Muss, H.; Nielsen, M.E. Sarcopenia as a predictor of complications and survival following radical cystectomy. J. Urol. 2014, 191, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Psutka, S.P.; Carrasco, A.; Schmit, G.D.; Moynagh, M.R.; Boorjian, S.A.; Frank, I.; Stewart, S.B.; Thapa, P.; Tarrell, R.F.; Cheville, J.C.; et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer 2014, 120, 2910–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirasawa, Y.; Nakashima, J.; Yunaiyama, D.; Sugihara, T.; Gondo, T.; Nakagami, Y.; Horiguchi, Y.; Ohno, Y.; Namiki, K.; Ohori, M.; et al. Sarcopenia as a Novel Preoperative Prognostic Predictor for Survival in Patients with Bladder Cancer Undergoing Radical Cystectomy. Ann. Surg. Oncol. 2016, 23, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Morizawa, Y.; Hori, S.; Marugami, N.; Shimada, K.; Gotoh, D.; Tatsumi, Y.; Nakai, Y.; Inoue, T.; Anai, S.; et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer 2017, 17, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh-Maeda, Y.; Kawahara, T.; Miyoshi, Y.; Tsutsumi, S.; Takamoto, D.; Shimokihara, K.; Hayashi, Y.; Mochizuki, T.; Ohtaka, M.; Nakamura, M.; et al. A low psoas muscle volume correlates with a longer hospitalization after radical cystectomy. BMC Urol. 2017, 17, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, R.; Gierth, M.; Zeman, F.; Reiffen, M.; Seeger, P.; Wezel, F.; Pycha, A.; Comploj, E.; Bonatti, M.; Ritter, M.; et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Yokoyama, M.; Nakanishi, Y.; Tobisu, K.; Koga, F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE 2015, 10, e0115895. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Akamatsu, N.; Nakagawa, T.; Gonoi, W.; Kanatani, A.; Miyazaki, H.; Fujimura, T.; Fukuhara, H.; Kume, H.; Homma, Y. Sarcopenia Evaluated Using the Skeletal Muscle Index Is a Significant Prognostic Factor for Metastatic Urothelial Carcinoma. Clin. Genitourin. Cancer 2016, 14, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, R.; Kawahara, T.; Ohtake, S.; Saitoh, Y.; Tsutsumi, S.; Teranishi, J.I.; Miyoshi, Y.; Nakaigawa, N.; Yao, M.; Kobayashi, K.; et al. A Low Psoas Muscle Index before Treatment Can Predict a Poorer Prognosis in Advanced Bladder Cancer Patients Who Receive Gemcitabine and Nedaplatin Therapy. BioMed Res. Int. 2017, 2017, 7981549. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Takei, K.; Uematsu, T.; Tokura, Y.; Suzuki, I.; Sakamoto, K.; Nishihara, D.; Yamaguchi, Y.; Mizuno, T.; Nukui, A.; et al. Significance of sarcopenia as a prognostic factor for metastatic urothelial carcinoma patients treated with systemic chemotherapy. Int. J. Clin. Oncol. 2018, 23, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Zargar, H.; Almassi, N.; Kovac, E.; Ercole, C.; Remer, E.; Rini, B.; Stephenson, A.; Garcia, J.A.; Grivas, P. Change in Psoas Muscle Volume as a Predictor of Outcomes in Patients Treated with Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Bladder Cancer 2017, 3, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, H.; Kataoka, M.; Nakanishi, Y.; Sakamoto, K.; Takemura, K.; Suzuki, H.; Ito, M.; Tobisu, K.I.; Fujii, Y.; Koga, F. Posttherapeutic skeletal muscle mass recovery predicts favorable prognosis in patients with advanced urothelial carcinoma receiving first-line platinum-based chemotherapy. Urol. Oncol. 2018, 36, 156.e9–156.e16. [Google Scholar] [CrossRef] [PubMed]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.M.; O’Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Park, H.K. Connecting Myokines and Metabolism. Endocrinol. Metab. 2015, 30, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, V.C.; Martin, P.; Lewandowski, P.A. Cancer cachexia: Impact, mechanisms and emerging treatments. J. Cachexia Sarcopenia Muscle 2013, 4, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.S.; Smith, H.J.; Drake, J.L.; Tisdale, M.J. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res. 2001, 61, 3604–3609. [Google Scholar] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Muzio, G.; Ricci, M.; Traverso, N.; Monacelli, F.; Oraldi, M.; Maggiora, M.; Canuto, R.A. 4-Hydroxyhexenal and 4-hydroxynonenal are mediators of the anti-cachectic effect of n-3 and n-6 polyunsaturated fatty acids on human lung cancer cells. Free. Radic. Boil. Med. 2016, 99, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Cockbain, A.J.; Volpato, M.; Race, A.D.; Munarini, A.; Fazio, C.; Belluzzi, A.; Loadman, P.M.; Toogood, G.J.; Hull, M.A. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 2014, 63, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Gomes-Marcondes, M.C. Metformin treatment modulates the tumour-induced wasting effects in muscle protein metabolism minimising the cachexia in tumour-bearing rats. BMC Cancer 2016, 16, 418. [Google Scholar] [CrossRef] [PubMed]
- Beluzi, M.; Peres, S.B.; Henriques, F.S.; Sertie, R.A.; Franco, F.O.; Santos, K.B.; Knobl, P.; Andreotti, S.; Shida, C.S.; Neves, R.X.; et al. Pioglitazone treatment increases survival and prevents body weight loss in tumor-bearing animals: Possible anti-cachectic effect. PLoS ONE 2015, 10, e0122660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dionne, I.J.; Kinaman, K.A.; Poehlman, E.T. Sarcopenia and muscle function during menopause and hormone-replacement therapy. J. Nutr. Health Aging 2000, 4, 156–161. [Google Scholar] [PubMed]
- Stene, G.B.; Helbostad, J.L.; Balstad, T.R.; Riphagen, I.I.; Kaasa, S.; Oldervoll, L.M. Effect of physical exercise on muscle mass and strength in cancer patients during treatment—A systematic review. Crit. Rev. Oncol. Hematol. 2013, 88, 573–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, B.; Steindorf, K.; Wiskemann, J.; Ulrich, C.M. Impact of resistance training in cancer survivors: A meta-analysis. Med. Sci. Sports Exerc. 2013, 45, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.W.; Lahart, I.; Carmichael, A.R.; Koutedakis, Y.; Metsios, G.S. Cancer cachexia prevention via physical exercise: Molecular mechanisms. J. Cachexia Sarcopenia Muscle 2013, 4, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Cardinale, D.A.; Norrbom, J.; Chapman, M.; Ivarsson, N.; Wengstrom, Y.; Sundberg, C.J.; Rundqvist, H. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology 2015, 29, 908–920, 922. [Google Scholar] [PubMed]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar] [CrossRef] [PubMed]

| Study (Year) | Country | No. of Total Patients | No. of Sarcopenic Patients | Cancer Type | Therapeutic Interventions | Definition of Sarcopenia | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| Smith et al. (2014) | United States | 200 | 77 (39%) | Bladder cancer | Radical cystectomy | TPA † < 653 cm2/m2 for males and <523 cm2/m2 for females | The Kaplan–Meier curves showed no significant association between OS and sarcopenia (p = 0.36). | [45] |
| Psutka et al. (2014) | United States | 205 | 141 (69%) | Bladder cancer | Radical cystectomy | SMI < 55 cm2/m2 for males and <39 cm2/m2 for females | Sarcopenia was an independent poor prognostic factor with HR 2.14 for CSS (p = 0.007) and 1.93 for OS (p = 0.004). | [46] |
| Hirasawa et al. (2016) | Japan | 136 | 65 (48%) | Bladder cancer | Radical cystectomy | SMI < 43 cm2/m2 for males with BMI < 25 cm2/m2, <53 cm2/m2 for males with BMI ≥ 25 cm2/m2, and <41 cm2/m2 for females | Sarcopenia was an independent poor prognostic factor with HR 2.3 for CSS (p = 0.015). | [47] |
| Miyake et al. (2017) | Japan | 89 | 22 (25%) | Bladder cancer | Radical cystectomy | SMI < 43 cm2/m2 for males with BMI < 25 cm2/m2, <53 cm2/m2 for males with BMI ≥ 25 cm2/m2, and <41 cm2/m2 for females | Sarcopenia was an independent poor prognostic factor with HR 2.2 for OS (p = 0.03). | [48] |
| Saitoh-Maeda et al. (2018) | Japan | 63 (male only) | 141 (69%) | Bladder cancer | Radical cystectomy | PMI < 400 cm2/m2 | In male patients, non-sarcopenic patients had a significantly better OS than sarcopenic counterparts (2,889 vs. 2,009 days; p = 0.023). | [49] |
| Mayr et al. (2018) | Germany | 500 | 189 (38%) | Bladder cancer | Radical cystectomy | SMI < 43 cm2/m2 for males with BMI < 25 cm2/m2, <53 cm2/m2 for males with BMI ≥ 25 cm2/m2, and <41 cm2/m2 for females | Sarcopenia was an independent poor prognostic factor with HR 1.42 for CSS (p = 0.048) and 1.43 for OS (p = 0.01). | [50] |
| Fukushima et al. (2015) | Japan | 88 | 67 (76%) | Advanced urothelial carcinoma | Miscellaneous | SMI < 43 cm2/m2 for males with BMI < 25 cm2/m2, <53 cm2/m2 for males with BMI ≥ 25 cm2/m2, and <41 cm2/m2 for females | Sarcopenia was an independent poor prognostic factor with HR 3.36 for OS (p < 0.001). | [51] |
| Taguchi et al. (2015) | Japan | 100 | Not reported | Metastatic urothelial carcinoma | Systemic chemotherapy | SMI < 55 cm2/m2 for males and <39 cm2/m2 for females | Sarcopenia was an independent poor prognostic factor with HR 2.07 for CSS (p = 0.045). | [52] |
| Kasahara et al. (2017) | Japan | 27 | 14 (52%) | Advanced bladder cancer | Systemic chemotherapy | PMI < 2.49 cm2/m2 for males and <2.07 cm2/m2 for females | The OS of the non-sarcopenic group was significantly better than that of the sarcopenic group (561 vs. 223 days; p = 0.0150). | [53] |
| Abe et al. (2018) | Japan | 87 | Not reported | Metastatic urothelial carcinoma | Systemic chemotherapy | SMI < 55 cm2/m2 for males and <39 cm2/m2 for females | Sarcopenia was not significantly associated with OS (p = 0.11). SMI stratified by BMI was an independent predictor for shorter OS (p = 0.026). | [54] |
| Study (Year) | Country | No. of Total Patients | Cancer Type | Therapeutic Interventions | Evaluation of Skeletal Muscle Mass | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Miyake et al. (2017) | Japan | 89 | Bladder cancer | Radical cystectomy | Postoperative changes in psoas major muscle volume were calculated after a radical cystectomy. | A 10% loss in the volume of the psoas muscle was an independent poor prognostic factor with HR 2.4 for OS (p = 0.02). | [48] |
| Zargar et al. (2017) | United States | 60 | Bladder cancer | NAC and a radical cystectomy | Changes in PMV were calculated from pre- and post-NAC CT images. | The proportion of PMV decline during NAC was not a predictor of OS after a radical cystectomy (p = 0.85). | [55] |
| Fukushima et al. (2018) | Japan | 72 | Advanced urothelial carcinoma | Systemic chemotherapy | Changes in SMI were calculated from pretherapeutic and posttherapeutic CT images. | Post-therapeutic skeletal muscle mass recovery was an independent favorable prognostic factor with HR 0.24 for RFS (p < 0.001) and 0.21 for OS (p < 0.001). | [56] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, H.; Takemura, K.; Suzuki, H.; Koga, F. Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer. Int. J. Mol. Sci. 2018, 19, 2999. https://doi.org/10.3390/ijms19102999
Fukushima H, Takemura K, Suzuki H, Koga F. Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer. International Journal of Molecular Sciences. 2018; 19(10):2999. https://doi.org/10.3390/ijms19102999
Chicago/Turabian StyleFukushima, Hiroshi, Kosuke Takemura, Hiroaki Suzuki, and Fumitaka Koga. 2018. "Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer" International Journal of Molecular Sciences 19, no. 10: 2999. https://doi.org/10.3390/ijms19102999






