Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-κB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice
Abstract
:1. Introduction
2. Results
2.1. Identification of Phytochemicals in EEP
2.2. Effects of EEP on Symptoms of DSS-Induced Colitis Mice
2.3. Effects of EEP on Histological Changes in DSS-Induced Colitis Mice
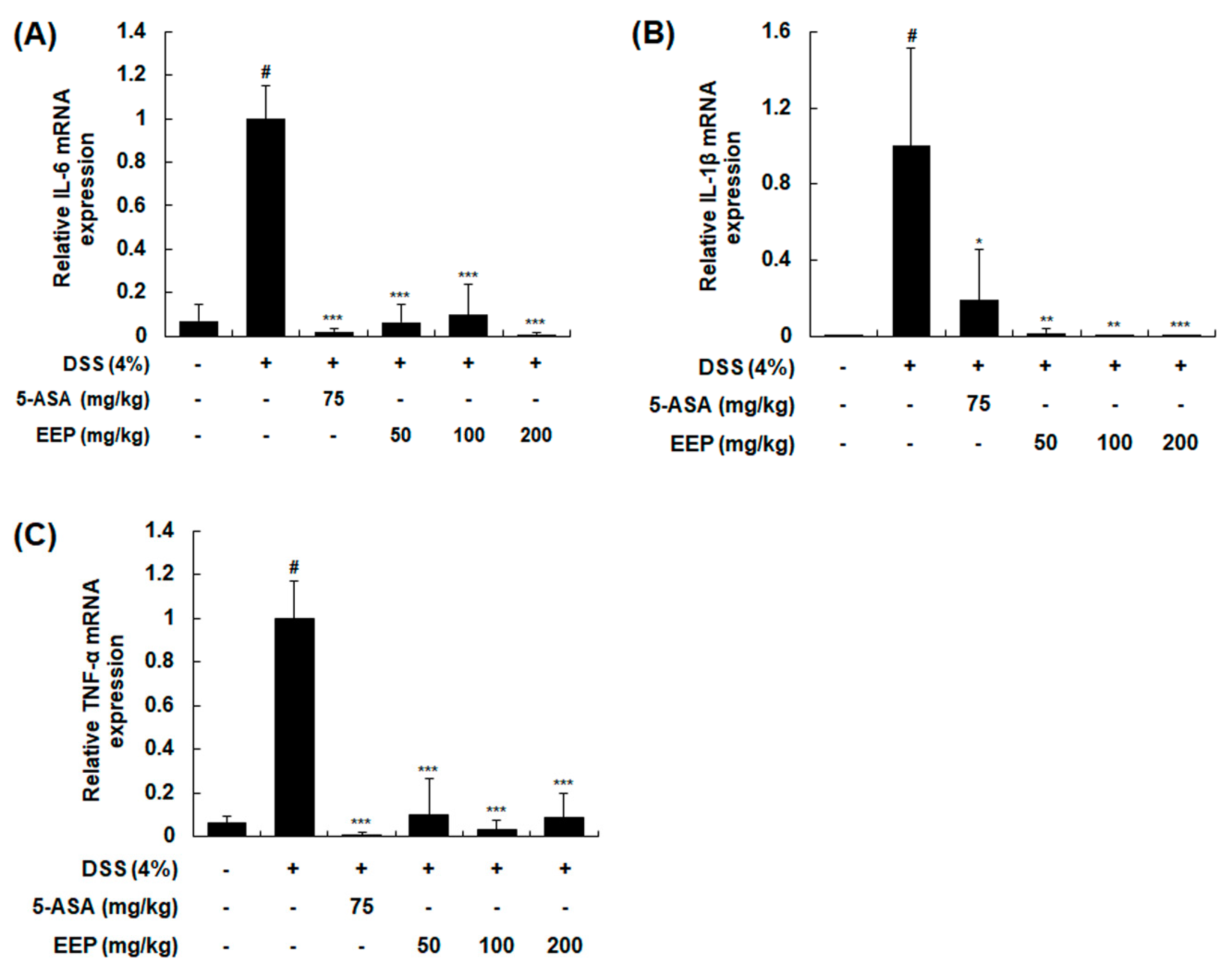
2.4. Effects of EEP on mRNA Expression of Pro-Inflammatory Cytokines in DSS-Induced Colitis Mice
2.5. Effects of EEP on Protein Expression of Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase (COX)-2 and the Activation of NF-κB and STAT3 in DSS-Induced Colitis Mice
2.6. Effects of EEP on LPS-Induced Production of NO and PGE2 by Suppressing Expression of iNOS and COX-2 in RAW 264.7 Macrophages
2.7. Effects of EEP on LPS-Induced Production and mRNA Expression Levels of Pro-Inflammatory Cytokines in RAW 264.7 Macrophages
2.8. Effects of EEP on Activation of NF-κB and STAT3 in RAW 264.7 Macrophages
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Ethanol Extract of Persea americana Mill.
4.3. Sample Preparation and HPLC-PDA-ESI-MS Analysis
4.4. Measurement of Total Phenolic Content
4.5. The Measurement of Total Flavonoid Content
4.6. Cell Culture
4.7. MTT Assay
4.8. Measurement of NO and Cytokines
4.9. Animals
4.10. Induction of Colitis
4.11. Evaluation of DAI
4.12. Western Blot Analysis
4.13. Histopathology
4.14. Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR)
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COX-2 | cyclooxygenase-2 |
| DAI | disease activity index |
| DSS | dextran sulfate sodium |
| EEP | ethanol extract of Persea americana Mill. |
| IBD | inflammatory bowel disease |
| iNOS | inducible nitric oxide synthase |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| NF-κB | nuclear factor-kappa B |
| STAT3 | signal transducer and activator of transcription 3 |
| TNF-α | tumor necrosis factor-α |
References
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, J.; Baldassano, R.N. Inflammatory bowel disease: The difference between children and adults. Inflamm. Bowel Dis. 2008, 14 (Suppl. 2), S9–S11. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 2008, 8, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, H.; Wang, T.; Shi, F. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int. Immunopharmacol. 2017, 50, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef]
- Karin, M. Inflammation-activated protein kinases as targets for drug development. Proc. Am. Thorac. Soc. 2005, 2, 386–390. [Google Scholar] [CrossRef]
- Han, Y.M.; Koh, J.; Kim, J.W.; Lee, C.; Koh, S.J.; Kim, B.; Lee, K.L.; Im, J.P.; Kim, J.S. NF-κB activation correlates with disease phenotype in Crohn’s disease. PLoS ONE 2017, 12, e0182071. [Google Scholar]
- Lovato, P.; Brender, C.; Agnholt, J.; Kelsen, J.; Kaltoft, K.; Svejgaard, A.; Eriksen, K.W.; Woetmann, A.; Odum, N. Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J. Biol. Chem. 2003, 278, 16777–16781. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.P.; Gao, K.; Byrns, R.; Heber, D. California Hass avocado: Profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar] [CrossRef] [PubMed]
- Carranza, J.; Alvizouri, M.; Alvarado, M.R.; Chavez, F.; Gomez, M.; Herrera, J.E. Effects of avocado on the level of blood lipids in patients with phenotype II and IV dyslipidemias. Arch. Inst. Cardiol. Mex. 1995, 65, 342–348. [Google Scholar] [PubMed]
- Colquhoun, D.M.; Moores, D.; Somerset, S.M.; Humphries, J.A. Comparison of the effects on lipoproteins and apolipoproteins of a diet high in monounsaturated fatty acids, enriched with avocado, and a high-carbohydrate diet. Am. J. Clin. Nutr. 1992, 56, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, W.C. Influence of avocados on serum cholesterol. Proc. Soc. Exp. Biol. Med. 1960, 104, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, G.; Meretski, S.; Segal, J.; Tarshis, M.; Schroeder, A.; Zanin-Zhorov, A.; Lion, G.; Ingber, A.; Hochberg, M. Polyhydroxylated fatty alcohols derived from avocado suppress inflammatory response and provide non-sunscreen protection against UV-induced damage in skin cells. Arch. Dermatol. Res. 2011, 303, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Gabay, O.; Gosset, M.; Levy, A.; Salvat, C.; Sanchez, C.; Pigenet, A.; Sautet, A.; Jacques, C.; Berenbaum, F. Stress-induced signaling pathways in hyalin chondrocytes: Inhibition by Avocado-Soybean Unsaponifiables (ASU). Osteoarthr. Cartil. 2008, 16, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Piraud, M.; Vianey-Saban, C.; Petritis, K.; Elfakir, C.; Steghens, J.P.; Morla, A.; Bouchu, D. ESI-MS/MS analysis of underivatised amino acids: A new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun. Mass Spectrom. 2003, 17, 1297–1311. [Google Scholar] [CrossRef]
- Liu, R.; Ye, Y.; Qiang, L.; Liao, X.; Zhao, Y. The fragmentation pathway of the nucleosides under the electrospray ionization multi-stage mass spectrometry. Life Sci. J. 2008, 5, 37–40. [Google Scholar]
- Hurtado-Fernandez, E.; Pacchiarotta, T.; Gomez-Romero, M.; Schoenmaker, B.; Derks, R.; Deelder, A.M.; Mayboroda, O.A.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A. Ultra high performance liquid chromatography-time of flight mass spectrometry for analysis of avocado fruit metabolites: Method evaluation and applicability to the analysis of ripening degrees. J. Chromatogr. A 2011, 1218, 7723–7738. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Schwerd, T.; Koletzko, S.; Shah, N.; Kammermeier, J.; Elkadri, A.; Ouahed, J.; Wilson, D.C.; Travis, S.P.; Turner, D.; et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014, 147, 990–1007.e3. [Google Scholar] [CrossRef]
- Pereira, J.L.; Hughes, L.E.; Young, H.L. Spleen size in patients with inflammatory bowel disease. Does it have any clinical significance? Dis. Colon Rectum 1987, 30, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Shalkami, A.S.; Hassan, M.; Bakr, A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2018, 37, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Faunce, D.E.; Stacey, M.; Terajewicz, A.; Nakamura, T.; Zhang-Hoover, J.; Kerley, M.; Mucenski, M.L.; Gordon, S.; Stein-Streilein, J. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J. Exp. Med. 2005, 201, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.Q.; Targan, S.R. Immunopathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Sklyarov, A.Y.; Panasyuk, N.B.; Fomenko, I.S. Role of nitric oxide-synthase and cyclooxygenase/lipooxygenase systems in development of experimental ulcerative colitis. J. Physiol. Pharmacol. 2011, 62, 65–73. [Google Scholar] [PubMed]
- Dawn, B.; Xuan, Y.T.; Guo, Y.; Rezazadeh, A.; Stein, A.B.; Hunt, G.; Wu, W.J.; Tan, W.; Bolli, R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc. Res. 2004, 64, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, L.; Lee, T.J. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-kappaB activation. Biochem. Pharmacol. 2000, 59, 1445–1457. [Google Scholar] [CrossRef]
- Gren, S.T.; Grip, O. Role of Monocytes and Intestinal Macrophages in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1992–1998. [Google Scholar] [CrossRef]
- Yu, Q.; Zeng, K.; Ma, X.; Song, F.; Jiang, Y.; Tu, P.; Wang, X. Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-κB and JNK/p38 MAPK signaling pathways. Int. Immunopharmacol. 2016, 38, 104–114. [Google Scholar] [CrossRef]
- Sakurai, H.; Nishi, A.; Sato, N.; Mizukami, J.; Miyoshi, H.; Sugita, T. TAK1-TAB1 fusion protein: A novel constitutively active mitogen-activated protein kinase kinase kinase that stimulates AP-1 and NF-κB signaling pathways. Biochem. Biophys. Res. Commun. 2002, 297, 1277–1281. [Google Scholar] [CrossRef]
- Liu, Y.W.; Ong, W.K.; Su, Y.W.; Hsu, C.C.; Cheng, T.H.; Tsai, Y.C. Anti-inflammatory effects of Lactobacillus brevis K65 on RAW 264.7 cells and in mice with dextran sulphate sodium-induced ulcerative colitis. Benef. Microbes 2016, 7, 387–396. [Google Scholar] [CrossRef] [PubMed]
- De Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Kate, J.H.; van Nifterick, W. The design of a saccade-size predictor for eye communication. Med. Prog. Technol. 1988, 13, 179–193. [Google Scholar] [PubMed]
- Knight-Sepulveda, K.; Kais, S.; Santaolalla, R.; Abreu, M.T. Diet and Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2015, 11, 511–520. [Google Scholar]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar]
- Evaldsson, C.; Ryden, I.; Uppugunduri, S. Anti-inflammatory effects of exogenous uridine in an animal model of lung inflammation. Int. Immunopharmacol. 2007, 7, 1025–1032. [Google Scholar] [CrossRef]
- Pirchl, M.; Ullrich, C.; Sperner-Unterweger, B.; Humpel, C. Homocysteine has anti-inflammatory properties in a hypercholesterolemic rat model in vivo. Mol. Cell. Neurosci. 2012, 49, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Souza, D.G.; Bobermin, L.D.; Goncalves, C.A.; Souza, D.O.; Quincozes-Santos, A. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal. 2015, 11, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, R.; Ballester, I.; Lopez-Posadas, R.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; Sanchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M. Intestinal epithelium in inflammatory bowel disease. Front. Med. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.S.; Tortori, C.J.; Castelo-Branco, M.T.; Carvalho, A.T.; Margallo, V.S.; Delgado, C.F.; Dines, I.; Elia, C.C. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: Evidence of altered expression of FasL and perforin cytotoxic pathways. Int. J. Colorectal Dis. 2005, 20, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.Q.; Schulze-Osthoff, K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Isidro, R.A.; Appleyard, C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G59–G73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wera, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A. Neutrophil expression and infiltration into Crohn’s intestine. Saudi J. Gastroenterol. 2005, 11, 63–72. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Rodriguez, D.; Russo, M.; Campa, A. Macrophage activation includes high intracellular myeloperoxidase activity. Biochem. Biophys. Res. Commun. 2002, 292, 869–873. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. The monocyte-macrophage axis in the intestine. Cell. Immunol. 2014, 291, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Singer, I.I.; Kawka, D.W.; Schloemann, S.; Tessner, T.; Riehl, T.; Stenson, W.F. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998, 115, 297–306. [Google Scholar] [CrossRef]
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Misyak, S.; Guri, A.J.; Hontecillas, R. PPAR gamma is highly expressed in F4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell. Immunol. 2009, 258, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejardin, E. The alternative NF-κB pathway from biochemistry to biology: Pitfalls and promises for future drug development. Biochem. Pharmacol. 2006, 72, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Pettersson, S.; Meyer zum Buschenfelde, K.H.; Strober, W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates established experimental colitis in mice. Nat. Med. 1996, 2, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.P.; Mark, K.G.; Leslie, K.; Pao, W.; Motoi, N.; Gerald, W.L.; Travis, W.D.; Bornmann, W.; Veach, D.; Clarkson, B.; et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J. Clin. Investig. 2007, 117, 3846–3856. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Herrmann, A.; Deng, J.H.; Kujawski, M.; Niu, G.; Li, Z.; Forman, S.; Jove, R.; Pardoll, D.M.; Yu, H. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell 2009, 15, 283–293. [Google Scholar] [CrossRef]
- Li, Z.; Wong, A.; Henning, S.M.; Zhang, Y.; Jones, A.; Zerlin, A.; Thames, G.; Bowerman, S.; Tseng, C.H.; Heber, D. Hass avocado modulates postprandial vascular reactivity and postprandial inflammatory responses to a hamburger meal in healthy volunteers. Food Funct. 2013, 4, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Compound | Rt (min) | Precursor Ion (m/z) | Molecular Formula | λmax (nm) |
|---|---|---|---|---|
| 1. Homocysteine | 7.70 | 136.04344 [M + H]+ | C4H9NO2S | 264 |
| 2. Guanine | 8.20 | 152.04841 [M + H]+ | C5H5N5O | 253 |
| 3. Cytidine | 8.68 | 244.09982 [M + H]+ 112.02048 [M-glu + H]+ | C9H13N3O5 | 215, 279 |
| 4. Uridine | 18.03 | 245.08380 [M + H]+ 267.06869 [M + Na]+ | C9H12N2O6 | 261 |
| 5. Guanosine | 24.02 | 284.10544 [M + H]+ 152.04486 [M-glu + H]+ | C10H13N5O5 | 253 |
| Total Polyphenol | Total Flavonoid | |
|---|---|---|
| Contents (mg/g) | 4.52 ± 0.01 | 1.56 ± 0.05 |
| DAI Score | Weight Loss (%) | Stool Consistency | Occult/Gross Bleeding |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1–5 | Loose stools | Hemoccult positive |
| 2 | 5–10 | ||
| 3 | 10–20 | ||
| 4 | >20 | Diarrhea | Gross bleeding |
| Gene | Sequence | |
|---|---|---|
| IL-6 | Forward | GAGGATACCACTCCCAACAGACC |
| Reverse | AAGTGCATCATCGTTGTTCATACA | |
| IL-1β | Forward | ACCTGCTGGTGTGTGACGTT |
| Reverse | TCGTTGCTTGGTTCTCCTTG | |
| TNF-α | Forward | AGCACAGAAAGCATGATCCG |
| Reverse | CTGATGAGAGGGAGGCCATT | |
| iNOS | Forward | AATGGCAACATCAGGTCGGCCATCACT |
| Reverse | GCTGTGTGTCACAGAAGTCTCGAACTC | |
| COX-2 | Forward | GGAGAGACTATCAAGA TAGT |
| Reverse | ATGGTCAGTAGACTTTTACA | |
| F4/80 | Forward | AGGACTGGAAGCCCATAGCCAA |
| Reverse | GCATCTAGCAATGGACAGCTG | |
| β-actin | Forward | ATCACTATTGGCAACGAGCG |
| Reverse | ATCACTATTGGCAACGAGCG | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.Y.; Chung, K.-S.; Shin, J.-S.; Park, G.; Jang, Y.P.; Lee, K.-T. Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-κB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice. Int. J. Mol. Sci. 2019, 20, 177. https://doi.org/10.3390/ijms20010177
Hong JY, Chung K-S, Shin J-S, Park G, Jang YP, Lee K-T. Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-κB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice. International Journal of Molecular Sciences. 2019; 20(1):177. https://doi.org/10.3390/ijms20010177
Chicago/Turabian StyleHong, Joo Young, Kyung-Sook Chung, Ji-Sun Shin, Geonha Park, Young Pyo Jang, and Kyung-Tae Lee. 2019. "Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-κB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice" International Journal of Molecular Sciences 20, no. 1: 177. https://doi.org/10.3390/ijms20010177
APA StyleHong, J. Y., Chung, K.-S., Shin, J.-S., Park, G., Jang, Y. P., & Lee, K.-T. (2019). Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-κB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice. International Journal of Molecular Sciences, 20(1), 177. https://doi.org/10.3390/ijms20010177