Impact of pH Modification on Protein Polymerization and Structure–Function Relationships in Potato Protein and Wheat Gluten Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protein Extractability in pH-Modified Protein Powders, Films and Composites
2.2. Protein Extractability in Pressed Composites
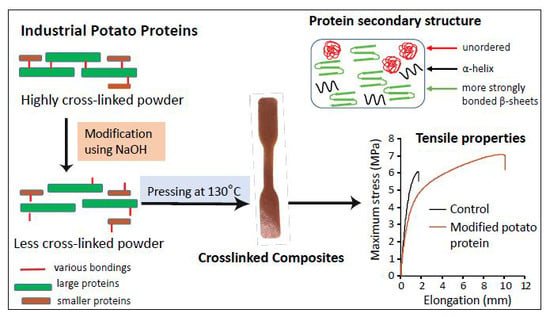
2.3. Effect of Chemical Modification on Secondary Structure of Proteins
2.4. Effect of Protein Modification on Mechanical Performance of the Pressed Composites
3. Materials and Methods
3.1. Wheat Gluten and Potato Proteins
3.2. Protein Modification
3.3. Sample Preparation and Compression Molding
3.4. SE-HPLC to Assess Protein Polymerization in Processed Composites
3.5. Tensile Testing
3.6. Fourier Transform Infrared Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ex | Extraction |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| HMw | High molecular weight proteins |
| LMw | Low molecular weight proteins |
| MPP | Modified potato protein |
| MWG | Modified wheat gluten |
| PP | Potato protein |
| SE-HPLC | Size Exclusion High Performance Liquid Chromatography |
| Tg | Glass transition temperature |
| WG | Wheat gluten |
References
- Kuktaite, R.; Newson, W.R.; Rasheed, F.; Plivelic, T.S.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Monitoring Nanostructure Dynamics and Polymerization in Glycerol Plasticized Wheat Gliadin and Glutenin Films: Relation to Mechanical Properties. ACS Sustain. Chem. Eng. 2016, 4, 2998–3007. [Google Scholar] [CrossRef]
- Newson, W.R.; Rasheed, F.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Plivelic, T.S.; Johansson, E. Commercial potato protein concentrate as a novel source for thermoformed bio-based plastic films with unusual polymerisation and tensile properties. RSC Adv. 2015, 5, 32217–32226. [Google Scholar] [CrossRef]
- Neurath, H.; Greenstein, J.P.; Putnam, F.W.; Erickson, J.A. The chemistry of protein denaturation. Chem. Rev. 1944, 34, 157–265. [Google Scholar] [CrossRef]
- Du, Y.; Chen, F.; Zhang, Y.; Rempel, C.; Thompson, M.R.; Liu, Q. Potato protein isolate-based biopolymers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Rasheed, F.; Hedenqvist, M.S.; Kuktaite, R.; Plivelic, T.S.; Gällstedt, M.; Johansson, E. Mild gluten separation–A non-destructive approach to fine tune structure and mechanical behavior of wheat gluten films. Ind. Crops Prod. 2015, 73, 90–98. [Google Scholar] [CrossRef]
- Van Koningsveld, G.A.; Gruppen, H.; de Jongh, H.H.J.; Wijngaards, G.; van Boekel, M.A.J.S.; Walstra, P.; Voragen, A.G.J. Effects of pH and Heat Treatments on the Structure and Solubility of Potato Proteins in Different Preparations. J. Agric. Food Chem. 2001, 49, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Ullsten, N.H.; Cho, S.-W.; Spencer, G.; Gallstedt, M.; Johansson, E.; Hedenqvist, M.S. Properties of Extruded Vital Wheat Gluten Sheets with Sodium Hydroxide and Salicylic Acid. Biomacromolecules 2009, 10, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Pommet, M.; Morel, M.-H.; Redl, A.; Guilbert, S. Aggregation and degradation of plasticized wheat gluten during thermo-mechanical treatments, as monitored by rheological and biochemical changes. Polymer 2004, 45, 6853–6860. [Google Scholar] [CrossRef]
- Olabarrieta, I.; Cho, S.W.; Gällstedt, M.; Sarasua, J.R.; Johansson, E.; Hedenqvist, M.S. Aging properties of films of plasticized vital wheat gluten cast from acidic and basic solutions. Biomacromolecules 2006, 7, 1657–1664. [Google Scholar] [CrossRef]
- Ture, H.; Gallstedt, M.; Kuktaite, R.; Johansson, E.; Hedenqvist, M.S. Protein network structure and properties of wheat gluten extrudates using a novel solvent-free approach with urea as a combined denaturant and plasticiser. Soft Matter 2011, 7, 9416–9423. [Google Scholar] [CrossRef]
- Rasheed, F.; Newson, W.R.; Plivelic, T.S.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Macromolecular changes and nano-structural arrangements in gliadin and glutenin films upon chemical modification: Relation to functionality. Int. J. Biol. Macromol. 2015, 79, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kuktaite, R.; Plivelic, T.S.; Cerenius, Y.; Hedenqvist, M.S.; Gallstedt, M.; Marttila, S.; Ignell, R.; Popineau, Y.; Tranquet, O.; Shewry, P.R.; et al. Structure and Morphology of Wheat Gluten Films: From Polymeric Protein Aggregates toward Superstructure Arrangements. Biomacromolecules 2011, 12, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Ullsten, N.H.; Gällstedt, M.; Spencer, G.M.; Johansson, E.; Marttila, S.; Ignell, R.; Hedenqvist, M.S. Extruded High Quality Materials From Wheat Gluten. Polym. Renew. Resour. 2010, 1, 173–186. [Google Scholar] [CrossRef]
- Gennadios, A.; Brandenburg, A.H.; Weller, C.L.; Testin, R.F. Effect of pH on properties of wheat gluten and soy protein isolate films. J. Agric. Food Chem. 1993, 41, 1835–1839. [Google Scholar] [CrossRef]
- Kuktaite, R.; Türe, H.; Hedenqvist, M.S.; Gällstedt, M.; Plivelic, T.S. Gluten Biopolymer and Nanoclay-Derived Structures in Wheat Gluten–Urea–Clay Composites: Relation to Barrier and Mechanical Properties. ACS Sustain. Chem. Eng. 2014, 2, 1439–1445. [Google Scholar] [CrossRef]
- Newson, W.R.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Effect of Additives on the Tensile Performance and Protein Solubility of Industrial Oilseed Residual Based Plastics. J. Agric. Food Chem. 2014, 62, 6707–6715. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Baraniak, B. Effects of plasticizers, pH and heating of film-forming solution on the properties of pea protein isolate films. J. Food Eng. 2011, 105, 295–305. [Google Scholar] [CrossRef]
- Domenek, S.; Morel, M.-H.; Bonicel, J.; Guilbert, S. Polymerization kinetics of wheat gluten upon thermosetting. A mechanistic model. J. Agric. Food Chem. 2002, 50, 5947–5954. [Google Scholar] [CrossRef]
- Gällstedt, M.; Mattozzi, A.; Johansson, E.; Hedenqvist, M.S. Transport and Tensile Properties of Compression-Molded Wheat Gluten Films. Biomacromolecules 2004, 5, 2020–2028. [Google Scholar] [CrossRef]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Markedal, K.E.; Petersen, I.L.; Sørensen, J.C.; Kuktaite, R. The impact of newly produced protein and dietary fiber rich fractions of yellow pea (Pisum sativum L.) on the structure and mechanical properties of pasta-like sheets. Food Res. Int. 2018, 106, 607–618. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Water and Glycerol as Plasticizers Affect Mechanical and Water Vapor Barrier Properties of an Edible Wheat Gluten Film. J. Food Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Johansson, E.; Malik, A.H.; Hussain, A.; Rasheed, F.; Newson, W.R.; Plivelic, T.; Hedenqvist, M.S.; Gällstedt, M.; Kuktaite, R. Wheat Gluten Polymer Structures: The Impact of Genotype, Environment, and Processing on Their Functionality in Various Applications. Cereal Chem. J. 2013, 90, 367–376. [Google Scholar] [CrossRef]
- Newson, W.R.; Kuktaite, R.; Hedenqvist, M.; Gällstedt, M.; Johansson, E. Oilseed Meal Based Plastics from Plasticized, Hot Pressed Crambe abyssinica and Brassica carinata Residuals. J. Am. Oil Chem. Soc. 2013, 90, 1229–1237. [Google Scholar] [CrossRef]
- Gerrard, J.A. Protein–protein crosslinking in food: Methods, consequences, applications. Trends Food Sci. Technol. 2002, 13, 391–399. [Google Scholar] [CrossRef]
- Lagrain, B.; Goderis, B.; Brijs, K.; Delcour, J.A. Molecular basis of processing wheat gluten toward biobased materials. Biomacromolecules 2010, 11, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, I.; Lagrain, B.; Delcour, J.A.; Türe, H.; Hedenqvist, M.S.; Johansson, E.; Kuktaite, R. Crosslinks in wheat gluten films with hexagonal close-packed protein structures. Ind. Crops Prod. 2013, 51, 229–235. [Google Scholar] [CrossRef]
- Giosafatto, C.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and Characterization of Bioplastics from Grass Pea Flour Cast in the Presence of Microbial Transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef]
- Ceresino, E.B.; de Melo, R.R.; Kuktaite, R.; Hedenqvist, M.S.; Zucchi, T.D.; Johansson, E.; Sato, H.H. Transglutaminase from newly isolated Streptomyces sp. CBMAI 1617: Production optimization, characterization and evaluation in wheat protein and dough systems. Food Chem. 2018, 241, 403–410. [Google Scholar] [CrossRef]
- Ceresino, E.B.; Kuktaite, R.; Sato, H.H.; Hedenqvist, M.S.; Johansson, E. Impact of gluten separation process and transglutaminase source on gluten based dough properties. Food Hydrocolloid. 2019, 87, 661–669. [Google Scholar] [CrossRef]
- Morel, M.H.; Redl, A.; Guilbert, S. Mechanism of heat and shear mediated aggregation of wheat gluten protein upon mixing. Biomacromolecules 2002, 3, 488–497. [Google Scholar] [CrossRef]
- Rasheed, F.; Newson, W.R.; Plivelic, T.S.; Kuktaite, R.; Hedenqvist, M.S.; Gallstedt, M.; Johansson, E. Structural architecture and solubility of native and modified gliadin and glutenin proteins: Non-crystalline molecular and atomic organization. RSC Adv. 2014, 4, 2051–2060. [Google Scholar] [CrossRef]
- Blomfeldt, T.O.J.; Kuktaite, R.; Johansson, E.; Hedenqvist, M.S. Mechanical Properties and Network Structure of Wheat Gluten Foams. Biomacromolecules 2011, 12, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Muneer, F.; Andersson, M.; Koch, K.; Menzel, C.; Hedenqvist, M.S.; Gallstedt, M.; Plivelic, T.S.; Kuktaite, R. Nanostructural Morphology of Plasticized Wheat Gluten and Modified Potato Starch Composites: Relationship to Mechanical and Barrier Properties. Biomacromolecules 2015, 16, 695–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowick, J.S. Exploring β-sheet structure and interactions with chemical model systems. Acc. Chem. Res. 2008, 41, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Critical Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Gällstedt, M.; Johansson, E.; Hedenqvist, M.S. Injection-molded nanocomposites and materials based on wheat gluten. Int. J. Biol. Macromol. 2011, 48, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomfeldt, T.O.; Kuktaite, R.; Plivelic, T.S.; Rasheed, F.; Johansson, E.; Hedenqvist, M.S. Novel freeze-dried foams from glutenin-and gliadin-rich fractions. RSC Adv. 2012, 2, 6617–6627. [Google Scholar] [CrossRef]
- Muneer, F.; Andersson, M.; Koch, K.; Hedenqvist, M.S.; Gällstedt, M.; Plivelic, T.S.; Menzel, C.; Rhazi, L.; Kuktaite, R. Innovative Gliadin/Glutenin and Modified Potato Starch Green Composites: Chemistry, Structure, and Functionality Induced by Processing. ACS Sustain. Chem. Eng. 2016, 4, 6332–6343. [Google Scholar] [CrossRef]
- Sun, S.; Song, Y.; Zheng, Q. Thermo-molded wheat gluten plastics plasticized with glycerol: Effect of molding temperature. Food Hydrocolloid. 2008, 22, 1006–1013. [Google Scholar] [CrossRef]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Gällstedt, M.; Newson, W.R. Preparation, Properties, Protein Cross-Linking and Biodegradability of Plasticizer-Solvent Free Hemp Fibre Reinforced Wheat Gluten, Glutenin, and Gliadin Composites. BioResources 2014, 9, 5246–5261. [Google Scholar] [CrossRef]






| Type | Abbreviation | WG (wt.%) | PP (wt.%) | MWG (wt.%) | MPP (wt.%) |
|---|---|---|---|---|---|
| Controls | WG/PP 30/70 WG/PP 50/50 WG/PP 70/30 | 30 50 70 | 70 50 30 | --- --- --- | --- --- --- |
| Only PP Modified | WG/MPP 30/70 WG/MPP 50/50 WG/MPP 70/30 | 30 50 70 | --- --- --- | --- --- --- | 70 50 30 |
| MWG/MPP Modified in Composite | MWG/MPP 30/70 MWG/MPP 50/50 MWG/MPP 70/30 | --- --- --- | --- --- --- | 30 50 70 | 70 50 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Kuktaite, R. Impact of pH Modification on Protein Polymerization and Structure–Function Relationships in Potato Protein and Wheat Gluten Composites. Int. J. Mol. Sci. 2019, 20, 58. https://doi.org/10.3390/ijms20010058
Muneer F, Johansson E, Hedenqvist MS, Plivelic TS, Kuktaite R. Impact of pH Modification on Protein Polymerization and Structure–Function Relationships in Potato Protein and Wheat Gluten Composites. International Journal of Molecular Sciences. 2019; 20(1):58. https://doi.org/10.3390/ijms20010058
Chicago/Turabian StyleMuneer, Faraz, Eva Johansson, Mikael S. Hedenqvist, Tomás S. Plivelic, and Ramune Kuktaite. 2019. "Impact of pH Modification on Protein Polymerization and Structure–Function Relationships in Potato Protein and Wheat Gluten Composites" International Journal of Molecular Sciences 20, no. 1: 58. https://doi.org/10.3390/ijms20010058