Fighting Thyroid Cancer with Microgravity Research
Abstract
:1. Introduction
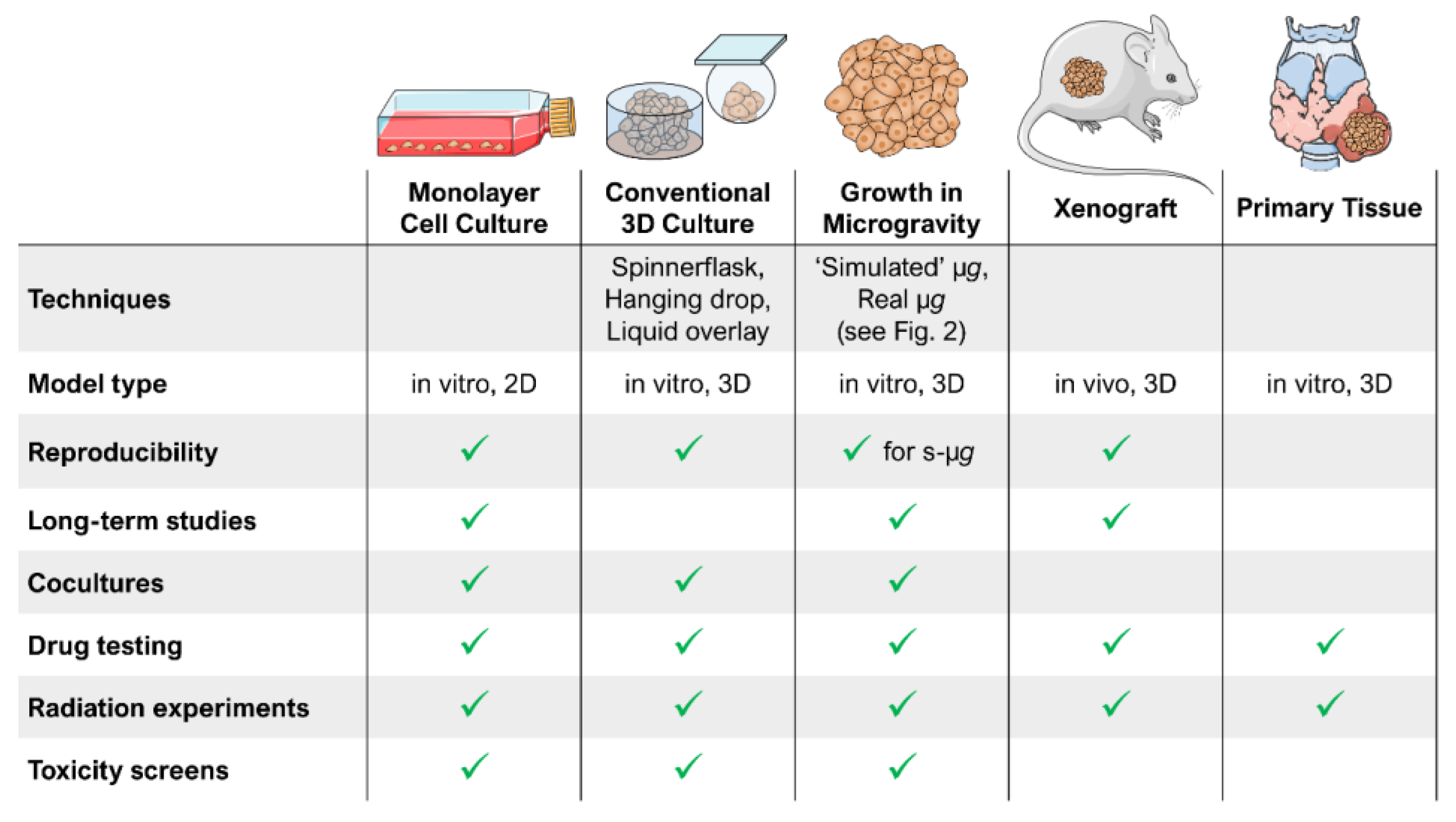
2. Ground-Based Techniques Allow Microgravity Research on Earth
3. The Behaviour of Normal Thyrocytes in Microgravity
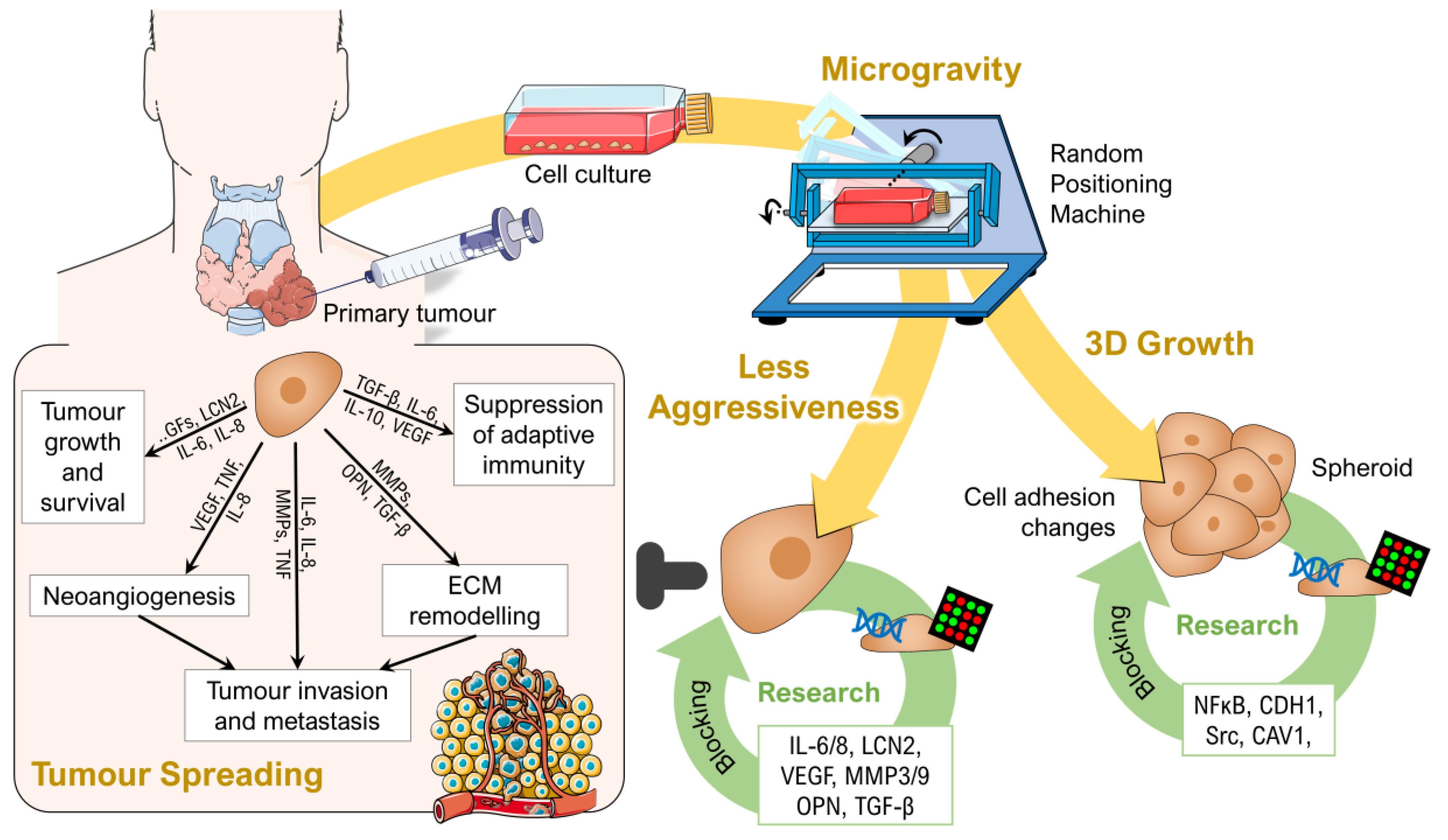
4. Research on Thyroid Cancer Cells in Microgravity
- Ground-Based Studies
- Parabolic and Sounding Rocket Flights
- Space Missions
- Supporting Semantic Analyses
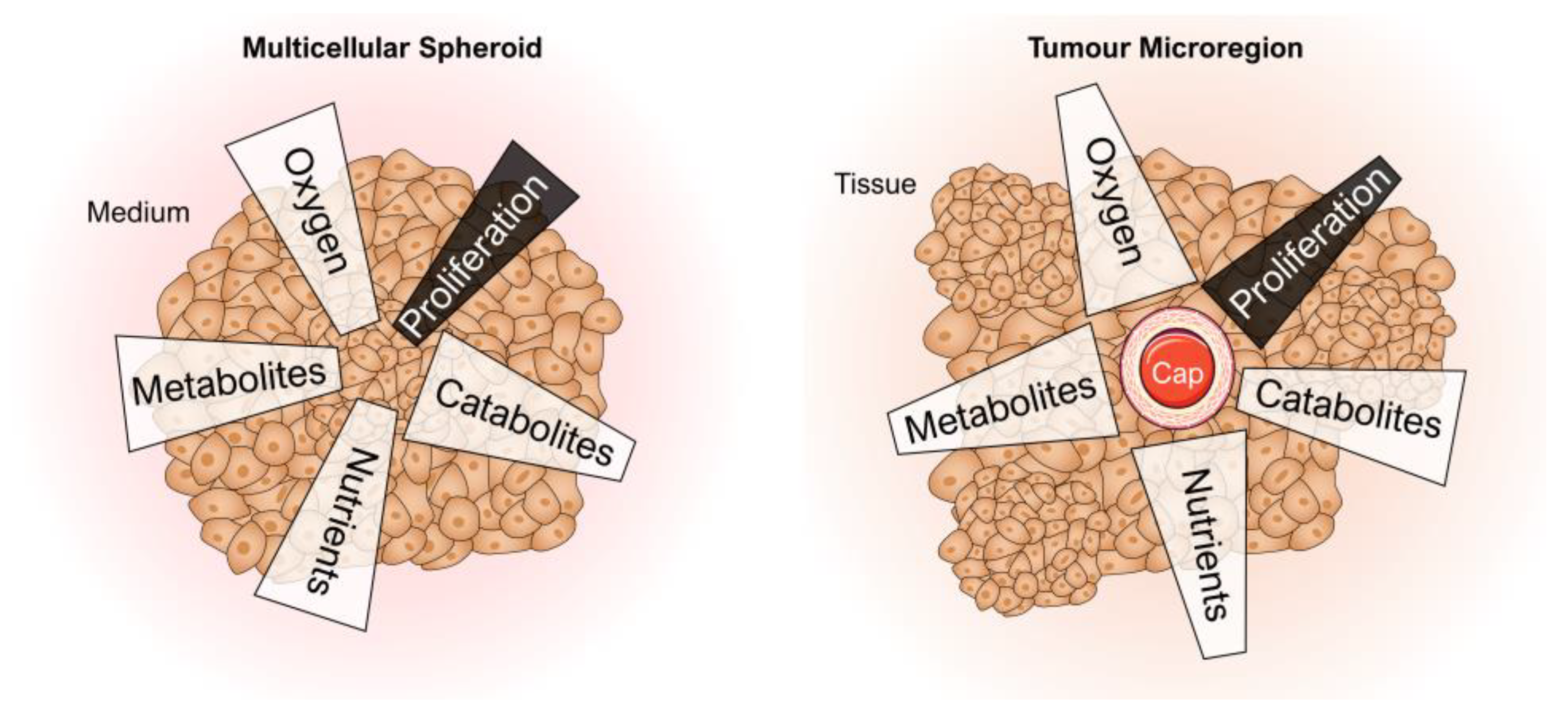
4.1. Spheroids as a 3D Tumour Model
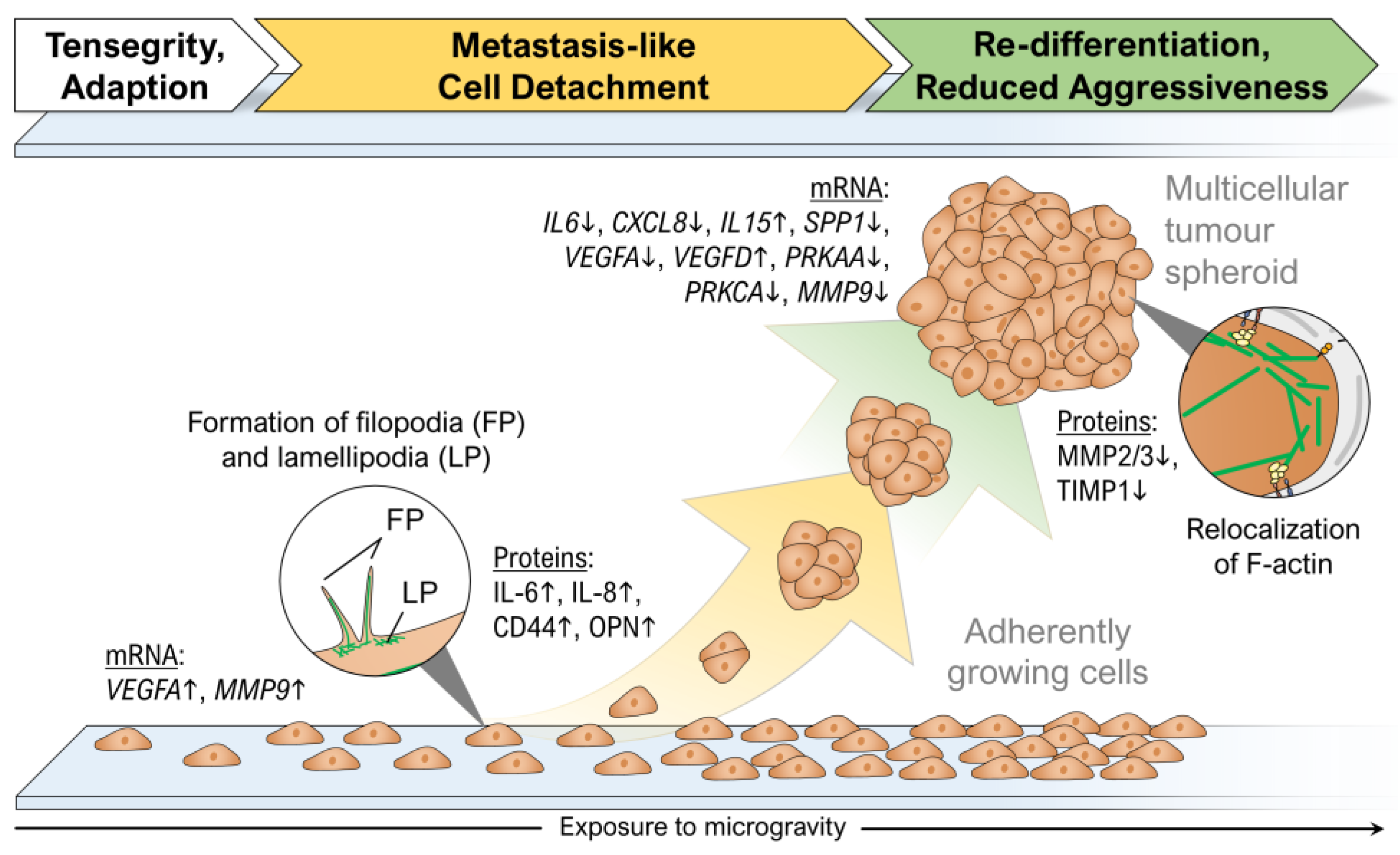
4.2. Growth of Thyroid Cancer Cells in Microgravity–A Temporary Mimicry of Metastasis?
4.3. Reduced Aggressiveness of Thyroid Cancer Cells after Long-Term Exposure to Real Microgravity
4.4. Drug Targeting
5. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ACTB | β-Actin |
| AD | Adherent cells |
| AMPK | 5′Adenosine monophosphate-activated protein kinase |
| CAV1/2 | Caveolin-1, -2, |
| CD44 | Cluster of differentiation 44 |
| CTGF | Connective tissue growth factor |
| CXCL | C-X-C motif |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ERK1/2 | Extracellular signal-regulated kinase 1,2 |
| FGF17 | Fibroblast growth factor 17 |
| FLK1 | Fetal liver kinase 1 |
| FLUMIAS | Fluorescence-microscopic analysis systems for space application |
| FLT1 | Fms-related tyrosine kinase 1 |
| FN1 | Fibronectin |
| FRC | Fast-rotating clinostat |
| IL | Interleukin |
| KDR | Kinase insert domain receptor |
| MCS | Multicellular spheroid |
| MMP | Matrix metalloproteinase |
| µg | Microgravity |
| Nek2 | Serine/threonine-protein kinase Nek2 |
| NFκB | Nuclear factor κ-light-chain-enhancer of activated B cells |
| OPN | Osteopontin |
| PRKAA | Protein kinase, AMP-activated, α catalytic subunit |
| PRKCA | Protein kinase Cα |
| RhoA | Ras homolog gene family, member A |
| RPM | Random positioning machine |
| RWV | Rotating wall vessel |
| SOX | Sex determining region Y-related high-mobility group box proteins |
| TGF-β | Transforming growth factor β |
| TIMP1 | Tissue inhibitor of metalloproteinases 1 |
| TLN1 | Talin 1 |
| TSH | Thyroid-stimulating hormone |
| TUBB | β-tubulin |
| VEGF | Vascular endothelial growth factor |
References
- Becker, J.L.; Souza, G.R. Using space-based investigations to inform cancer research on earth. Nat. Rev. Cancer 2013, 13, 315–327. [Google Scholar] [CrossRef]
- Jhala, D.V.; Kale, R.K.; Singh, R.P. Microgravity alters cancer growth and progression. Curr. Cancer Drug Targets 2014, 14, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Benes, E.; O’Reilly, K.C.; Wolf, D.A.; Linnehan, R.M.; Taher, A.; Kaysen, J.H.; Allen, P.L.; Goodwin, T.J. Mechanical culture conditions effect gene expression: Gravity-induced changes on the space shuttle. Physiol. Genom. 2000, 3, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2d vs. 3d cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Groebe, K.; Mueller-Klieser, W. On the relation between size of necrosis and diameter of tumor spheroids. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 395–401. [Google Scholar] [CrossRef]
- Kopp, S.; Warnke, E.; Wehland, M.; Aleshcheva, G.; Magnusson, N.E.; Hemmersbach, R.; Corydon, T.J.; Bauer, J.; Infanger, M.; Grimm, D. Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci. Rep. 2015, 5, 16691. [Google Scholar] [CrossRef] [Green Version]
- Aleshcheva, G.; Bauer, J.; Hemmersbach, R.; Slumstrup, L.; Wehland, M.; Infanger, M.; Grimm, D. Scaffold-free tissue formation under real and simulated microgravity conditions. Basic Clin. Pharmacol. Toxicol. 2016, 119, 26–33. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3d tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Al-Ramadan, A.; Mortensen, A.C.; Carlsson, J.; Nestor, M.V. Analysis of radiation effects in two irradiated tumor spheroid models. Oncol. Lett. 2018, 15, 3008–3016. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Albi, E.; Krüger, M.; Hemmersbach, R.; Lazzarini, A.; Cataldi, S.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Impact of gravity on thyroid cells. Int. J. Mol. Sci. 2017, 18, 972. [Google Scholar] [CrossRef] [PubMed]
- Braddock, M. From target identification to drug development in space: Using the microgravity assist. Curr. Drug Discov. Technol. 2019. [Google Scholar] [CrossRef]
- Eiermann, P.; Kopp, S.; Hauslage, J.; Hemmersbach, R.; Gerzer, R.; Ivanova, K. Adaptation of a 2-d clinostat for simulated microgravity experiments with adherent cells. Microgravity Sci. Technol. 2013, 25, 153–159. [Google Scholar] [CrossRef]
- Klaus, D.M. Clinostats and bioreactors. Gravit. Space Biol. Bull. 2001, 14, 55–64. [Google Scholar]
- Borst, A.G.; van Loon, J.J.W.A. Technology and developments for the random positioning machine, rpm. Microgravity Sci. Technol. 2008, 21, 287. [Google Scholar] [CrossRef]
- van Loon, J.J.W.A. Some history and use of the random positioning machine, rpm, in gravity related research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. Biomed. Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef]
- Albi, E.; Curcio, F.; Lazzarini, A.; Floridi, A.; Cataldi, S.; Lazzarini, R.; Loreti, E.; Ferri, I.; Ambesi-Impiombato, F.S. How microgravity changes galectin-3 in thyroid follicles. Biomed. Res. Int 2014, 2014, 5. [Google Scholar] [CrossRef]
- Masini, M.A.; Albi, E.; Barmo, C.; Bonfiglio, T.; Bruni, L.; Canesi, L.; Cataldi, S.; Curcio, F.; D’Amora, M.; Ferri, I.; et al. The impact of long-term exposure to space environment on adult mammalian organisms: A study on mouse thyroid and testis. PLoS ONE 2012, 7, e35418. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Ambesi-Impiombato, F.S.; Peverini, M.; Damaskopoulou, E.; Fontanini, E.; Lazzarini, R.; Curcio, F.; Perrella, G. Thyrotropin receptor and membrane interactions in frtl-5 thyroid cell strain in microgravity. Astrobiology 2011, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kossmehl, P.; Shakibaei, M.; Cogoli, A.; Infanger, M.; Curcio, F.; Schönberger, J.; Eilles, C.; Bauer, J.; Pickenhahn, H.; Schulze-Tanzil, G.; et al. Weightlessness induced apoptosis in normal thyroid cells and papillary thyroid carcinoma cells via extrinsic and intrinsic pathways. Endocrinology 2003, 144, 4172–4179. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Zhou, A.; Gordon, R.E.; Henderson, S.C.; Schwartz, A.E.; Schwartz, A.E.; Friedman, E.W.; Davies, T.F. Thyroid organoid formation in simulated microgravity: Influence of keratinocyte growth factor. Thyroid 2000, 10, 481–487. [Google Scholar] [CrossRef]
- Warnke, E.; Kopp, S.; Wehland, M.; Hemmersbach, R.; Bauer, J.; Pietsch, J.; Infanger, M.; Grimm, D. Thyroid cells exposed to simulated microgravity conditions – comparison of the fast rotating clinostat and the random positioning machine. Microgravity Sci. Technol. 2016, 28, 247–260. [Google Scholar] [CrossRef]
- Warnke, E.; Pietsch, J.; Kopp, S.; Bauer, J.; Sahana, J.; Wehland, M.; Krüger, M.; Hemmersbach, R.; Infanger, M.; Lützenberg, R.; et al. Cytokine release and focal adhesion proteins in normal thyroid cells cultured on the random positioning machine. Cell Physiol. Biochem. 2017, 43, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Curcio, F.; Ambesi-Impiombato, F.S.; Perrella, G.; Coon, H.G. Long-term culture and functional characterization of follicular cells from adult normal human thyroids. Proc. Natl. Acad. Sci. USA 1994, 91, 9004–9008. [Google Scholar] [CrossRef]
- Lewis, M.L.; Reynolds, J.L.; Cubano, L.A.; Hatton, J.P.; Lawless, B.D.; Piepmeier, E.H. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (jurkat). FASEB J. 1998, 12, 1007–1018. [Google Scholar] [CrossRef]
- Uva, B.M.; Masini, M.A.; Sturla, M.; Bruzzone, F.; Giuliani, M.; Tagliafierro, G.; Strollo, F. Microgravity-induced apoptosis in cultured glial cells. Eur. J. Histochem. 2002, 46, 209–214. [Google Scholar] [CrossRef]
- Battista, N.; Meloni, M.A.; Bari, M.; Mastrangelo, N.; Galleri, G.; Rapino, C.; Dainese, E.; Agro, A.F.; Pippia, P.; Maccarrone, M. 5-lipoxygenase-dependent apoptosis of human lymphocytes in the international space station: Data from the roald experiment. FASEB J. 2012, 26, 1791–1798. [Google Scholar] [CrossRef]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Baatout, S.; Witzing, A.; Grosse, J.; Bauer, J.; Cogoli, A.; Faramarzi, S.; Derradji, H.; et al. Induction of three-dimensional assembly and increase in apoptosis of human endothelial cells by simulated microgravity: Impact of vascular endothelial growth factor. Apoptosis 2006, 11, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.R.; Mayall, E.S.; Jones, T.; Sheer, D.; McDermid, S.; Kendall-Taylor, P.; Wynford-Thomas, D. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br. J. Cancer 1989, 60, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vistejnova, L.; Safrankova, B.; Nesporova, K.; Slavkovsky, R.; Hermannova, M.; Hosek, P.; Velebny, V.; Kubala, L. Low molecular weight hyaluronan mediated cd44 dependent induction of il-6 and chemokines in human dermal fibroblasts potentiates innate immune response. Cytokine 2014, 70, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Razidlo, G.L.; Burton, K.M.; McNiven, M.A. Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small gtpase cdc42. J. Biol. Chem. 2018, 293, 11143–11153. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Kossmehl, P.; Shakibaei, M.; Schulze-Tanzil, G.; Pickenhahn, H.; Bauer, J.; Paul, M.; Cogoli, A. Effects of simulated microgravity on thyroid carcinoma cells. Life Space Life Earth 2002, 501, 39–42. [Google Scholar]
- Kossmehl, P.; Shakibaei, M.; Cogoli, A.; Pickenhahn, H.; Paul, M.; Grimm, D. Simulated microgravity induces programmed cell death in human thyroid carcinoma cells. J. Gravit. Physiol. 2002, 9, 295–296. [Google Scholar] [PubMed]
- Kopp, S.; Sahana, J.; Islam, T.; Petersen, A.G.; Bauer, J.; Corydon, T.J.; Schulz, H.; Saar, K.; Huebner, N.; Slumstrup, L.; et al. The role of nfkappab in spheroid formation of human breast cancer cells cultured on the random positioning machine. Sci. Rep. 2018, 8, 921. [Google Scholar] [CrossRef]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Schulze-Tanzil, G.; Cogoli, A.; Faramarzi, S.; Bauer, J.; Curcio, F.; Paul, M.; Grimm, D. Longterm conditions of mimicked weightlessness influences the cytoskeleton in thyroid cells. J. Gravit. Physiol. 2004, 11, P169–P172. [Google Scholar]
- Ulbrich, C.; Pietsch, J.; Grosse, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Hauslage, J.; Hemmersbach, R.; Braun, M.; et al. Differential gene regulation under altered gravity conditions in follicular thyroid cancer cells: Relationship between the extracellular matrix and the cytoskeleton. Cell Physiol. Biochem. 2011, 28, 185–198. [Google Scholar] [CrossRef]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Bauer, J.; Kossmehl-Zorn, S.; Cogoli, A.; Curcio, F.; Oksche, A.; Wehland, M.; Kreutz, R.; et al. Simulated weightlessness changes the cytoskeleton and extracellular matrix proteins in papillary thyroid carcinoma cells. Cell Tissue Res. 2006, 324, 267–277. [Google Scholar] [CrossRef]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.; van Loon, J.J. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014, 28, 536–547. [Google Scholar] [CrossRef]
- Bauer, J.; Wehland, M.; Pietsch, J.; Sickmann, A.; Weber, G.; Grimm, D. Annotated gene and proteome data support recognition of interconnections between the results of different experiments in space research. Microgravity Sci. Technol. 2016, 28, 357–365. [Google Scholar] [CrossRef]
- Riwaldt, S.; Bauer, J.; Wehland, M.; Slumstrup, L.; Kopp, S.; Warnke, E.; Dittrich, A.; Magnusson, N.E.; Pietsch, J.; Corydon, T.J.; et al. Pathways regulating spheroid formation of human follicular thyroid cancer cells under simulated microgravity conditions: A genetic approach. Int. J. Mol. Sci. 2016, 17, 528. [Google Scholar] [CrossRef]
- Pietsch, J.; Sickmann, A.; Weber, G.; Bauer, J.; Egli, M.; Wildgruber, R.; Infanger, M.; Grimm, D. A proteomic approach to analysing spheroid formation of two human thyroid cell lines cultured on a random positioning machine. Proteomics 2011, 11, 2095–2104. [Google Scholar] [CrossRef]
- Bauer, J.; Kopp, S.; Schlagberger, E.M.; Grosse, J.; Sahana, J.; Riwaldt, S.; Wehland, M.; Luetzenberg, R.; Infanger, M.; Grimm, D. Proteome analysis of human follicular thyroid cancer cells exposed to the random positioning machine. Int. J. Mol. Sci. 2017, 18, 546. [Google Scholar] [CrossRef]
- Warnke, E.; Pietsch, J.; Wehland, M.; Bauer, J.; Infanger, M.; Görög, M.; Hemmersbach, R.; Braun, M.; Ma, X.; Sahana, J.; et al. Spheroid formation of human thyroid cancer cells under simulated microgravity: A possible role of ctgf and cav1. Cell Commun. Signal. 2014, 12, 32. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of sox family members in solid tumours and metastasis. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Ma, X.; Pietsch, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Bauer, J.; Braun, M.; Schwarzwälder, A.; Segerer, J.; et al. Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J. 2014, 28, 813–835. [Google Scholar] [CrossRef]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schutte, A.; Mayer, T.; Hulsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef]
- Kopp, S.; Krüger, M.; Feldmann, S.; Oltmann, H.; Schütte, A.; Schmitz, B.; Bauer, J.; Schulz, H.; Saar, K.; Huebner, N.; et al. Thyroid cancer cells in space during the TEXUS-53 sounding rocket mission-the thyroid project. Sci. Rep. 2018, 8, 10355. [Google Scholar] [CrossRef]
- Kopp, S.; Krüger, M.; Bauer, J.; Wehland, M.; Corydon, T.J.; Sahana, J.; Nassef, M.Z.; Melnik, D.; Bauer, T.J.; Schulz, H.; et al. Microgravity affects thyroid cancer cells during the TEXUS-53 mission stronger than hypergravity. Int. J. Mol. Sci. 2018, 19, 4001. [Google Scholar] [CrossRef] [PubMed]
- Riwaldt, S.; Bauer, J.; Pietsch, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Corydon, T.J.; Infanger, M.; Grimm, D. The importance of caveolin-1 as key-regulator of three-dimensional growth in thyroid cancer cells cultured under real and simulated microgravity conditions. Int. J. Mol. Sci. 2015, 16, 28296–28310. [Google Scholar] [CrossRef]
- Riwaldt, S.; Pietsch, J.; Sickmann, A.; Bauer, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Aleshcheva, G.; Corydon, T.J.; Infanger, M.; et al. Identification of proteins involved in inhibition of spheroid formation under microgravity. Proteomics 2015, 15, 2945–2952. [Google Scholar] [CrossRef]
- Melnik, D.; Krüger, M.; Kopp, S.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. Microgravity-based modulation of VEGF expression in human thyroid carcinoma cells. Front. Physiol. 2019, 9. [Google Scholar] [CrossRef]
- Pietsch, J.; Riwaldt, S.; Bauer, J.; Sickmann, A.; Weber, G.; Grosse, J.; Infanger, M.; Eilles, C.; Grimm, D. Interaction of proteins identified in human thyroid cells. Int. J. Mol. Sci. 2013, 14, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Bauer, J.; Weber, G.; Nissum, M.; Westphal, K.; Egli, M.; Grosse, J.; Schönberger, J.; Eilles, C.; Infanger, M.; et al. Proteome analysis of thyroid cancer cells after long-term exposure to a random positioning machine. Microgravity Sci. Technol. 2011, 23, 381–390. [Google Scholar] [CrossRef]
- Pietsch, J.; Sickmann, A.; Weber, G.; Bauer, J.; Egli, M.; Wildgruber, R.; Infanger, M.; Grimm, D. Metabolic enzyme diversity in different human thyroid cell lines and their sensitivity to gravitational forces. Proteomics 2012, 12, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Grimm, D.; Gombocz, E. Semantic analysis of thyroid cancer cell proteins obtained from rare research opportunities. J. Biomed. Inform. 2017, 76, 138–153. [Google Scholar] [CrossRef]
- Bauer, J.; Wehland, M.; Infanger, M.; Grimm, D.; Gombocz, E. Semantic analysis of posttranslational modification of proteins accumulated in thyroid cancer cells exposed to simulated microgravity. Int. J. Mol. Sci. 2018, 19, 2257. [Google Scholar] [CrossRef]
- Bauer, J.; Cohly, H.H.P.; Sahana, J.; Grimm, D. Preparative enrichment of human tissue cells capable to change a site of growth in vitro or in vivo-recent developments. Prep. Biochem. Biotechnol. 2018, 48, 954–960. [Google Scholar] [CrossRef]
- Grosse, J.; Wehland, M.; Pietsch, J.; Schulz, H.; Saar, K.; Hübner, N.; Eilles, C.; Bauer, J.; Abou-El-Ardat, K.; Baatout, S.; et al. Gravity-sensitive signaling drives 3-dimensional formation of multicellular thyroid cancer spheroids. FASEB J. 2012, 26, 5124–5140. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A. Multicellular tumor spheroids: Intermediates between monolayer culture and in vivo tumor. Cell Biol. Int. 1999, 23, 157–161. [Google Scholar] [CrossRef]
- Pietsch, J.; Ma, X.; Wehland, M.; Aleshcheva, G.; Schwarzwälder, A.; Segerer, J.; Birlem, M.; Horn, A.; Bauer, J.; Infanger, M.; et al. Spheroid formation of human thyroid cancer cells in an automated culturing system during the shenzhou-8 space mission. Biomaterials 2013, 34, 7694–7705. [Google Scholar] [CrossRef]
- Svejgaard, B.; Wehland, M.; Ma, X.; Kopp, S.; Sahana, J.; Warnke, E.; Aleshcheva, G.; Hemmersbach, R.; Hauslage, J.; Grosse, J.; et al. Common effects on cancer cells exerted by a random positioning machine and a 2d clinostat. PLoS ONE 2015, 10, e0135157. [Google Scholar] [CrossRef]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Krüger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased e-cadherin in mcf7 human breast cancer cells forming multicellular spheroids exposed to simulated microgravity. Proteomics 2018, 18, e1800015. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-d cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen 2004, 9, 273–285. [Google Scholar] [CrossRef]
- Willis, R.A. Metastatic tumours in the thyreoid gland. Am. J. Pathol. 1931, 7, 187–208.183. [Google Scholar]
- Schmidbauer, B.; Menhart, K.; Hellwig, D.; Grosse, J. Differentiated thyroid cancer-treatment: State of the art. Int. J. Mol. Sci. 2017, 18, 1292. [Google Scholar] [CrossRef]
- Wang, L.Y.; Palmer, F.L.; Nixon, I.J.; Thomas, D.; Patel, S.G.; Shaha, A.R.; Shah, J.P.; Tuttle, R.M.; Ganly, I. Multi-organ distant metastases confer worse disease-specific survival in differentiated thyroid cancer. Thyroid 2014, 24, 1594–1599. [Google Scholar] [CrossRef]
- Parameswaran, R.; Shulin Hu, J.; Min En, N.; Tan, W.B.; Yuan, N.K. Patterns of metastasis in follicular thyroid carcinoma and the difference between early and delayed presentation. Ann. R. Coll. Surg. Engl. 2017, 99, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M. Cytological features of medullary thyroid carcinoma in ascitic effusion. Diagn Cytopathol. 2017, 45, 1030–1032. [Google Scholar] [CrossRef]
- Al Habyan, S.; Kalos, C.; Szymborski, J.; McCaffrey, L. Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene 2018, 37, 5127–5135. [Google Scholar] [CrossRef]
- Mueller-Klieser, W. Multicellular spheroids. A review on cellular aggregates in cancer research. J. Cancer Res. Clin. Oncol. 1987, 113, 101–122. [Google Scholar] [CrossRef]
- Hamilton, G. Multicellular spheroids as an in vitro tumor model. Cancer Lett. 1998, 131, 29–34. [Google Scholar] [CrossRef]
- Northcott, J.M.; Dean, I.S.; Mouw, J.K.; Weaver, V.M. Feeling stress: The mechanics of cancer progression and aggression. Front. Cell Dev. Biol. 2018, 6, 17. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Yu, H.; Mouw, J.K.; Weaver, V.M. Forcing form and function: Biomechanical regulation of tumor evolution. Trends Cell Biol. 2011, 21, 47–56. [Google Scholar] [CrossRef]
- Ingber, D.E.; Wang, N.; Stamenovic, D. Tensegrity, cellular biophysics and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef]
- Ingber, D. How cells (might) sense microgravity. FASEB J. 1999, 13, 3–15. [Google Scholar] [CrossRef]
- Dittrich, A.; Grimm, D.; Sahana, J.; Bauer, J.; Krüger, M.; Infanger, M.; Magnusson, N.E. Key proteins involved in spheroid formation and angiogenesis in endothelial cells after long-term exposure to simulated microgravity. Cell Physiol. Biochem. 2018, 45, 429–445. [Google Scholar] [CrossRef]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via fak/rhoa-regulated mtorc1 and ampk pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, R.; Tan, X.; Zhang, J.; Fan, C.; Zhao, Q.; Deng, Y.; Xu, A.; Lukong, K.E.; Genth, H.; et al. Simulated microgravity reduces focal adhesions and alters cytoskeleton and nuclear positioning leading to enhanced apoptosis via suppressing fak/rhoa-mediated mtorc1/nf-kappab and ERK1/2 pathways. Int. J. Mol. Sci. 2018, 19, 1994. [Google Scholar] [CrossRef]
- Deng, B.; Liu, R.; Tian, X.; Han, Z.; Chen, J. Simulated microgravity inhibits the viability and migration of glioma via fak/rhoa/rock and fak/nek2 signaling. In Vitro Cell Dev. Biol. Anim. 2019, 55, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Xu, H.; Guo, Y.; Jiang, X.; Liu, Y.; Li, K.; Pan, C.; Yuan, M.; Wang, J.; Li, T.; et al. Simulated microgravity alters the metastatic potential of a human lung adenocarcinoma cell line. In Vitro Cell Dev. Biol. Anim. 2013, 49, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 2007, 1773, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Joy, M.; Bhargava, R.; Gunsaulus, M.; Lakshman, N.; Miron-Mendoza, M.; Petroll, M.; Condeelis, J.; Wells, A.; Roy, P. Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis. Oncogene 2014, 33, 2065–2074. [Google Scholar] [CrossRef]
- Miao, J.W.; Liu, L.J.; Huang, J. Interleukin-6-induced epithelial-mesenchymal transition through signal transducer and activator of transcription 3 in human cervical carcinoma. Int. J. Oncol. 2014, 45, 165–176. [Google Scholar] [CrossRef]
- Klassen, L.M.B.; Chequin, A.; Manica, G.C.M.; Biembengut, I.V.; Toledo, M.B.; Baura, V.A.; de, O.P.F.; Ramos, E.A.S.; Costa, F.F.; de Souza, E.M.; et al. Mmp9 gene expression regulation by intragenic epigenetic modifications in breast cancer. Gene 2018, 642, 461–466. [Google Scholar] [CrossRef]
- Wai, P.Y.; Kuo, P.C. Osteopontin: Regulation in tumor metastasis. Cancer Metastasis Rev. 2008, 27, 103–118. [Google Scholar] [CrossRef]
- Luca, M.; Huang, S.; Gershenwald, J.E.; Singh, R.K.; Reich, R.; Bar-Eli, M. Expression of interleukin-8 by human melanoma cells up-regulates mmp-2 activity and increases tumor growth and metastasis. Am. J. Pathol. 1997, 151, 1105–1113. [Google Scholar]
- Liu, W.; Xu, J.; Wang, M.; Wang, Q.; Bi, Y.; Han, M. Tumor-derived vascular endothelial growth factor (vegf)-a facilitates tumor metastasis through the vegf-vegfr1 signaling pathway. Int. J. Oncol. 2011, 39, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Sottnik, J.L.; Theodorescu, D. Cd44: A metastasis driver and therapeutic target. Oncoscience 2016, 3, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Nersita, R.; Matrone, A.; Klain, M.; Scavuzzo, F.; Vitolo, G.; Abbondanza, C.; Carlino, M.V.; Giacco, V.; Amato, G.; Carella, C. Decreased serum vascular endothelial growth factor-d levels in metastatic patients with differentiated thyroid carcinoma. Clin. Endocrinol. 2012, 76, 142–146. [Google Scholar] [CrossRef]
- Hsueh, C.; Lin, J.D.; Wu, I.C.; Chao, T.C.; Yu, J.S.; Liou, M.J.; Yeh, C.J. Vascular endothelial growth factors and angiopoietins in presentations and prognosis of papillary thyroid carcinoma. J. Surg. Oncol. 2011, 103, 395–399. [Google Scholar] [CrossRef]
- Hauslage, J.; Cevik, V.; Hemmersbach, R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). NPJ Microgravity 2017, 3, 12. [Google Scholar] [CrossRef]
- Wuest, S.L.; Stern, P.; Casartelli, E.; Egli, M. Fluid dynamics appearing during simulated microgravity using random positioning machines. PLoS ONE 2017, 12, e0170826. [Google Scholar] [CrossRef]
- Pietsch, J.; Kussian, R.; Sickmann, A.; Bauer, J.; Weber, G.; Nissum, M.; Westphal, K.; Egli, M.; Grosse, J.; Schonberger, J.; et al. Application of free-flow ief to identify protein candidates changing under microgravity conditions. Proteomics 2010, 10, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Denison, C.; Huibregtse, J.M.; Gygi, S.; Krug, R.M. Human isg15 conjugation targets both ifn-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. USA 2005, 102, 10200–10205. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.J.; Lenschow, D.J. The antiviral activities of isg15. J. Mol. Biol. 2013, 425, 4995–5008. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Guo, S.; Wu, W.; Wang, J.; Wu, S.; He, S.; Wan, Y.; Nandakumar, K.S.; Chen, X.; Sun, N.; et al. Q63, a novel denv2 rdrp non-nucleoside inhibitor, inhibited denv2 replication and infection. J. Pharmacol. Sci. 2018, 138, 247–256. [Google Scholar] [CrossRef]
- Bektas, N.; Noetzel, E.; Veeck, J.; Press, M.F.; Kristiansen, G.; Naami, A.; Hartmann, A.; Dimmler, A.; Beckmann, M.W.; Knüchel, R.; et al. The ubiquitin-like molecule interferon-stimulated gene 15 (isg15) is a potential prognostic marker in human breast cancer. Breast Cancer Res. 2008, 10, R58. [Google Scholar] [CrossRef]
- Hanke, J.H.; Gardner, J.P.; Dow, R.L.; Changelian, P.S.; Brissette, W.H.; Weringer, E.J.; Pollok, B.A.; Connelly, P.A. Discovery of a novel, potent and src family-selective tyrosine kinase inhibitor. Study of lck- and fynt-dependent t cell activation. J. Biol. Chem. 1996, 271, 695–701. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, M.; Sharma, P.L. Ameliorative effect of daidzein: A caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. Indian J. Exp. Biol. 2012, 50, 28–34. [Google Scholar]
- Liu, M.; Hummer, B.T.; Li, X.; Hassel, B.A. Camptothecin induces the ubiquitin-like protein, isg15 and enhances isg15 conjugation in response to interferon. J. Interferon Cytokine Res. 2004, 24, 647–654. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Luo, J. High expression of rab-like 3 (rabl3) is associated with poor survival of patients with non-small cell lung cancer via repression of mapk8/9/10-mediated autophagy. Med. Sci. Monit. 2016, 22, 1582–1588. [Google Scholar] [CrossRef]
- Baeck, C.; Wehr, A.; Karlmark, K.R.; Heymann, F.; Vucur, M.; Gassler, N.; Huss, S.; Klussmann, S.; Eulberg, D.; Luedde, T.; et al. Pharmacological inhibition of the chemokine ccl2 (mcp-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2012, 61, 416–426. [Google Scholar] [CrossRef]
- Dai, Y.; Rahmani, M.; Dent, P.; Grant, S. Blockade of histone deacetylase inhibitor-induced rela/p65 acetylation and nf-kappab activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, xiap downregulation and c-jun n-terminal kinase 1 activation. Mol. Cell. Biol. 2005, 25, 5429–5444. [Google Scholar] [CrossRef]
- Zhang, J.; He, D.H.; Zajac-Kaye, M.; Hochwald, S.N. A small molecule fak kinase inhibitor, gsk2256098, inhibits growth and survival of pancreatic ductal adenocarcinoma cells. Cell Cycle 2014, 13, 3143–3149. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Meng, T.; Ma, L.P.; Tong, L.J.; Shen, J.K.; Wang, Y.Q.; Miao, Z.H. New microtubulin inhibitor mt189 suppresses angiogenesis via the jnk-vegf/vegfr2 signaling axis. Cancer Lett. 2018, 416, 57–65. [Google Scholar] [CrossRef]
- Wu, B.; Li, J.; Huang, D.; Wang, W.; Chen, Y.; Liao, Y.; Tang, X.; Xie, H.; Tang, F. Baicalein mediates inhibition of migration and invasiveness of skin carcinoma through ezrin in a431 cells. BMC Cancer 2011, 11, 527. [Google Scholar] [CrossRef]
- Kreutz, D.; Sinthuvanich, C.; Bileck, A.; Janker, L.; Muqaku, B.; Slany, A.; Gerner, C. Curcumin exerts its antitumor effects in a context dependent fashion. J. Proteom. 2018, 182, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Lindberg, O.R.; Kuhn, H.G. Radixin inhibition decreases adult neural progenitor cell migration and proliferation in vitro and in vivo. Front. Cell Neurosci. 2013, 7, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placencio, V.R.; Ichimura, A.; Miyata, T.; DeClerck, Y.A. Small molecule inhibitors of plasminogen activator inhibitor-1 elicit anti-tumorigenic and anti-angiogenic activity. PLoS ONE 2015, 10, e0133786. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, G.J.; Kim, E.J.; Park, M.K.; Byun, H.J.; Nam, S.; Lee, H.; Lee, C.H. Novel effects of sphingosylphosphorylcholine on invasion of breast cancer: Involvement of matrix metalloproteinase-3 secretion leading to wnt activation. Biochim. Biophys. Acta 2016, 1862, 1533–1543. [Google Scholar] [CrossRef]
- O’Shea, L.K.; Abdulkhalek, S.; Allison, S.; Neufeld, R.J.; Szewczuk, M.R. Therapeutic targeting of neu1 sialidase with oseltamivir phosphate (tamiflu(r)) disables cancer cell survival in human pancreatic cancer with acquired chemoresistance. Onco Targets Ther. 2014, 7, 117–134. [Google Scholar] [CrossRef]




| Cell Line | Condition | Findings | Ref. |
|---|---|---|---|
| FTC-133 | Space ISS (5d) preincubation (12d) | Factors involved in inhibition of 3D growth: caveolin-1, VCAM-1 and activated protein kinase Cα recruited in caveolae | [52] |
| FTC-133 | Space ISS (5d) preincubation (12d) | Proteins involved in the inhibition of 3D growth: extracellular matrix proteins, phosphorylated profilin 1 | [53] |
| FTC-133 | Space (10d) Shenzhou 8 | IL6, CXCL8, IL15, SPP1, VEGFA, VEGFD, FGF17, MMP2, MMP3, TIMP1, PRKAA and PRKCA | [48] |
| FTC-133 | Space (10d) Shenzhou 8 | CTGF and EGF | [63] |
| FTC-133 | RPM 3d, (2d) preincubation (5d) preincubation | Vinculin, paxillin, focal adhesion kinase 1 and adenine diphosphate (ADP)-ribosylation factor 6 | [45] |
| FTC-133, Nthy-ori 3-1 | RPM (14d) | VEGF, FLT-1. FLK-1, CD44, Copine 1, TGM2, IL-6, IL-8, IL-17, OPN, neutrophil gelatinase-associated lipocalin (NGAL, LCN2) | [6] |
| ML-1, RO82-W-1 | RPM (3d), FRC (7d) | ML-1 cells: elevated release of IL-6 and monocyte chemoattractant protein (MCP-1) | [64] |
| Pathway/Function | Genes |
|---|---|
| Cell adhesion | VCAM1, CD44, CDH1 |
| Angiogenesis | VEGFD, VEGFA, FLK1 |
| Apoptosis | TGFB1 |
| Caveolae | CAV1 |
| Extracellular matrix | SPP1, MMP2, MMP3, TIMP1, FN1, COL1A1 |
| Inflammation | IL6, CXCL8, IL17 |
| NFκB signalling | NFKB1 |
| Protein kinases | PRKAA, PRKCA |
| Cytoskeleton | ACTB, TUBB, FN1 |
| Microgravity (Detachment) | Metastases (Detachment) | |
|---|---|---|
| Physical Trigger | Lapse of gravity (tensegrity, mechanical stress) | Pressure from growing tumour |
| Cytoskeleton | Formation of filopodia and lamellipodia [49] | Formation of filopodia and lamellipodia [85] |
| PFN↑ [6], phosphorylated profilin-1 prevented MCS formation [43] | Profilin 1↓ [86] | |
| Cell Adhesion | Blockage of E-cadherin leads to enhanced spheroid formation of MCF-7 breast cancer cells [65] | E-cadherin↓ [87] |
| ECM | MMP9↑ [43]; OPN↑ [61] | MMP9↑ [88]; OPN↑ [89] |
| Cytokines | IL-6↑, IL-8↑ [6] | IL-6↑ [87]; IL-8 enhances metastatic potential [90] |
| Growth Factors | VEGF-A↑ [48] | VEGF↑ facilitates metastasis through the VEGF-VEGFR1 signalling pathway [91] |
| Others | CD44↑ [61] | CD44↑ [92] |
| Drug | Target | Ref. |
|---|---|---|
| PP2 (4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine) | Proto-oncogene tyrosine-protein kinase Src | [102] |
| Daidzein | Caveolin-1 | [103] |
| Camptothecin | Ubiquitin-like protein ISG15 | [104] |
| SP600125 | Mitogen-activated protein kinase 8 | [105] |
| mNOX-E36 | C-C motif chemokine 2 | [106] |
| Dexamethasone, BAY 11-7082 | NFκB p65 | [107] |
| GSK2256098, MPAP | Focal adhesion kinase 1 | [108] |
| MT189 | Paxillin | [109] |
| Baicalein | Ezrin | [110] |
| Curcumin | HMOX-1 | [111] |
| DX52-1 | Radixin | [112] |
| TM5441 | Plasminogen activator inhibitor 1 | [113] |
| UK370106 | Stromelysin, (MMP3) | [114] |
| Oseltamivir | Sialidase | [115] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, M.; Melnik, D.; Kopp, S.; Buken, C.; Sahana, J.; Bauer, J.; Wehland, M.; Hemmersbach, R.; Corydon, T.J.; Infanger, M.; et al. Fighting Thyroid Cancer with Microgravity Research. Int. J. Mol. Sci. 2019, 20, 2553. https://doi.org/10.3390/ijms20102553
Krüger M, Melnik D, Kopp S, Buken C, Sahana J, Bauer J, Wehland M, Hemmersbach R, Corydon TJ, Infanger M, et al. Fighting Thyroid Cancer with Microgravity Research. International Journal of Molecular Sciences. 2019; 20(10):2553. https://doi.org/10.3390/ijms20102553
Chicago/Turabian StyleKrüger, Marcus, Daniela Melnik, Sascha Kopp, Christoph Buken, Jayashree Sahana, Johann Bauer, Markus Wehland, Ruth Hemmersbach, Thomas J. Corydon, Manfred Infanger, and et al. 2019. "Fighting Thyroid Cancer with Microgravity Research" International Journal of Molecular Sciences 20, no. 10: 2553. https://doi.org/10.3390/ijms20102553