Tyroxine Hydroxylase-Positive Neuronal Cell Population is Increased by Temporal Dioxin Exposure at Early Stage of Differentiation from Human Embryonic Stem Cells
Abstract
:1. Introduction
2. Results
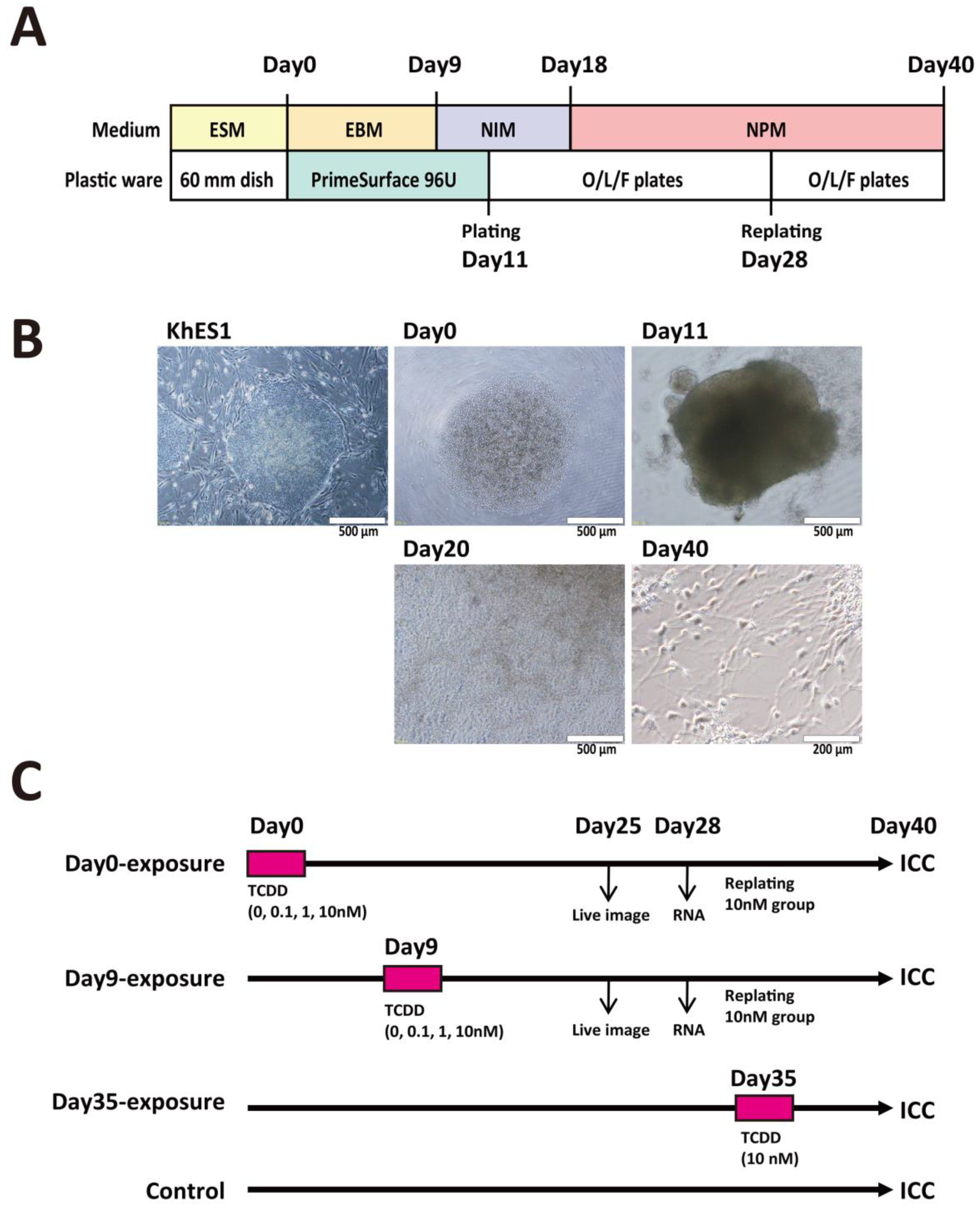
2.1. TCDD Exposure at EB Stage Increased Visible Neural Rosette Number
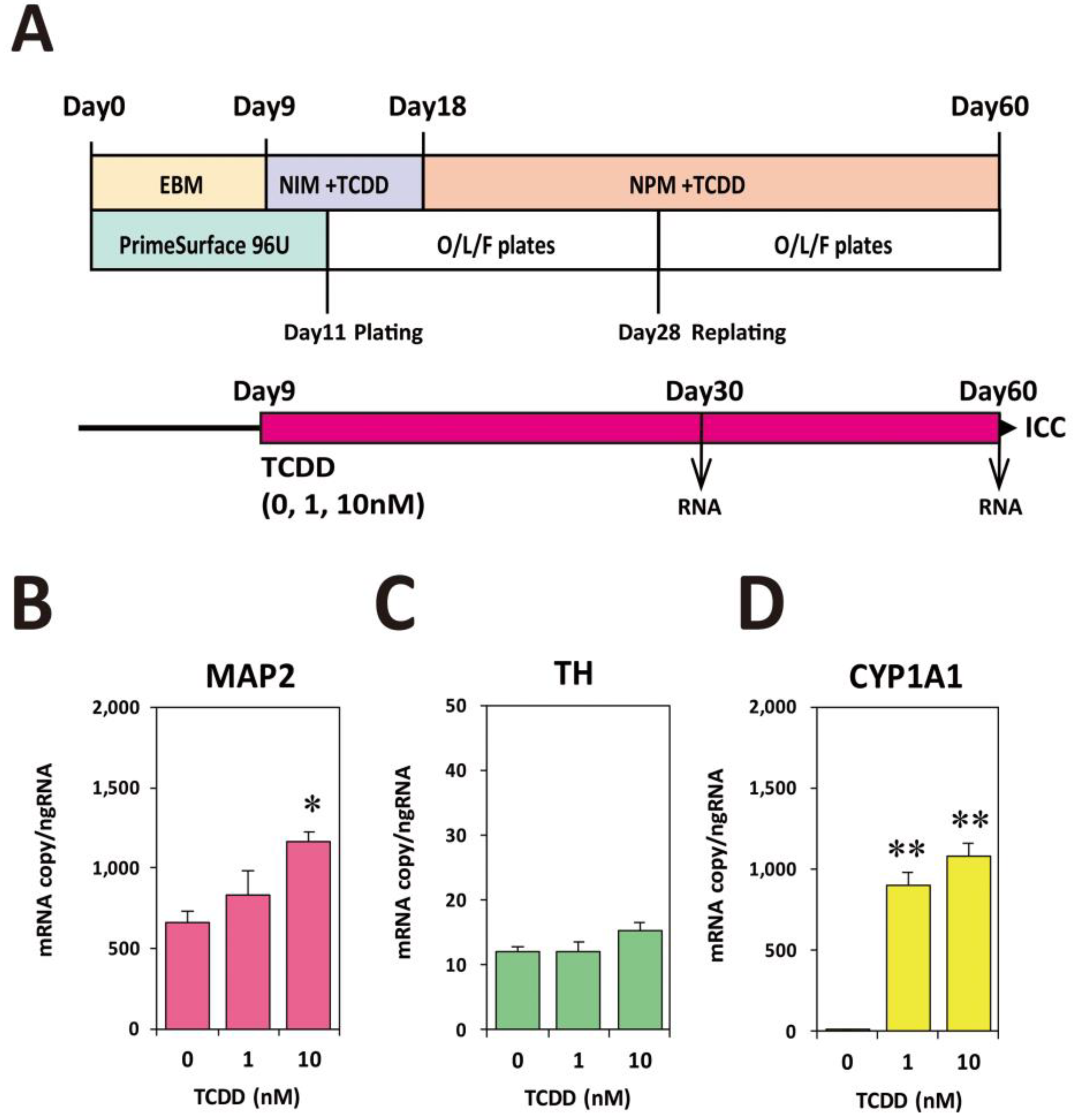
2.2. TCDD Exposure at EB Stage Increased Expression Levels of Neuronal Marker mRNAs
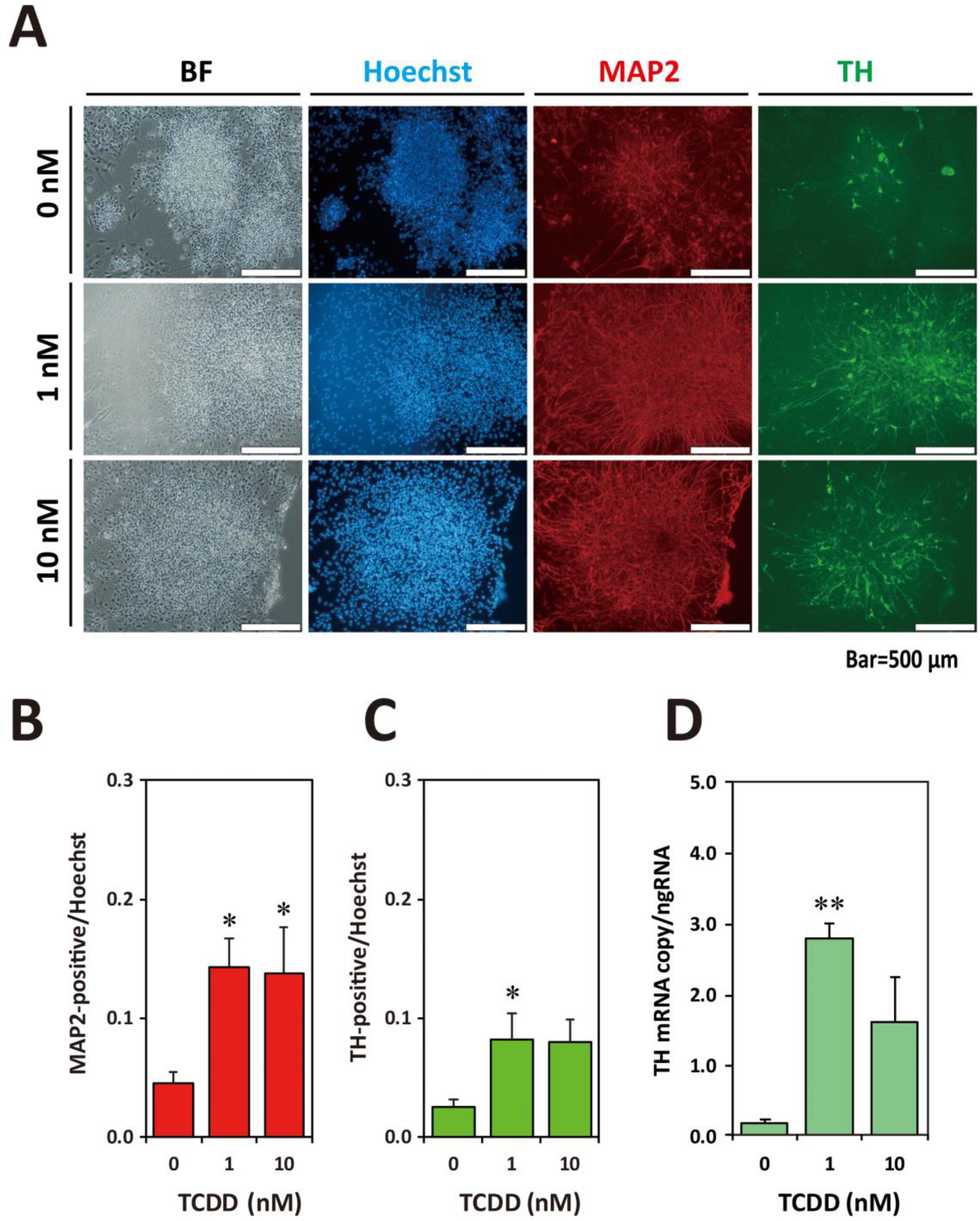
2.3. TCDD Exposure at EB Stage Increased Neuronal Cell Population
2.4. Rat Th-EGFP Trangene Did Not Work in the Human ESC-Derivatives
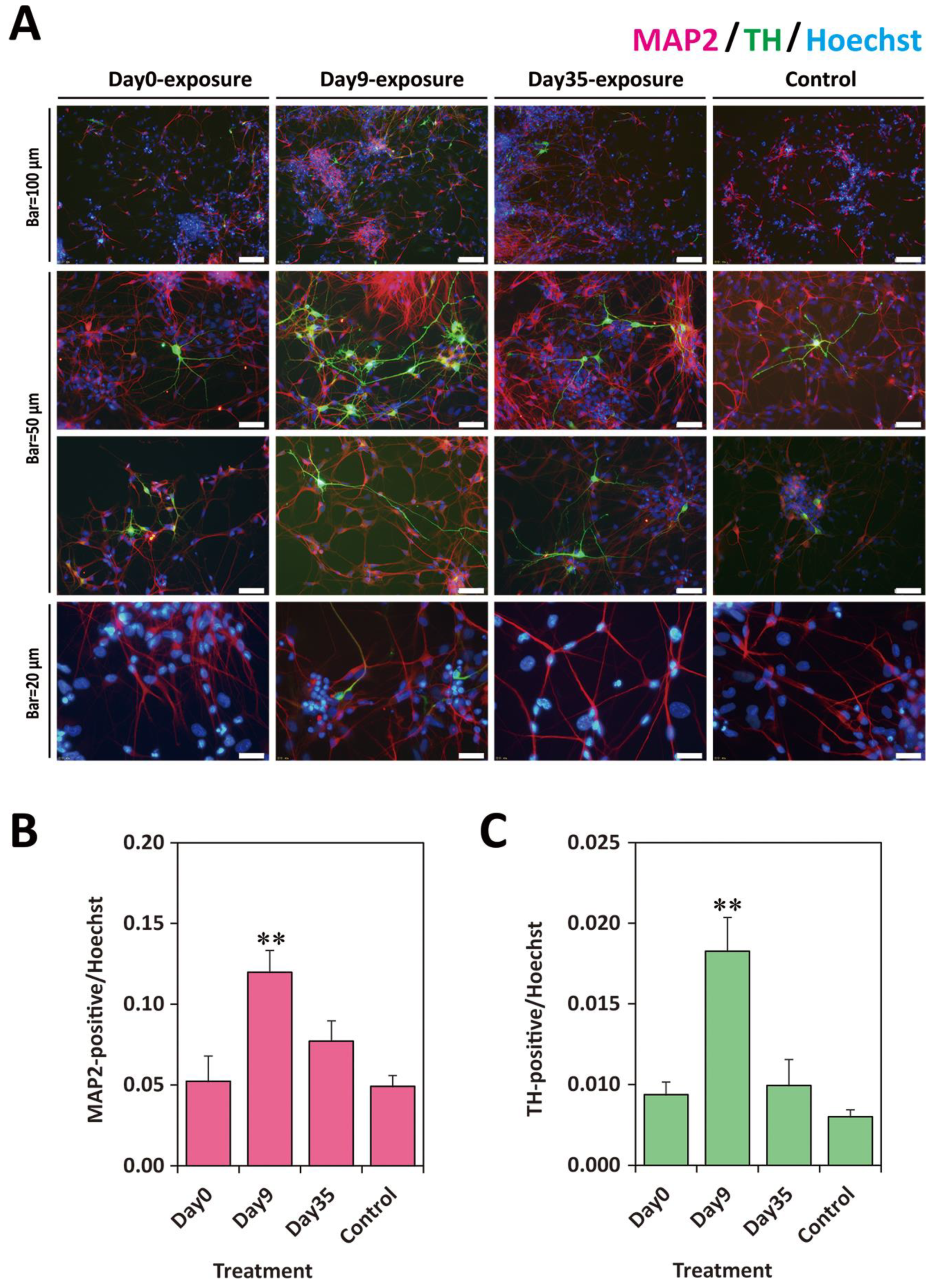
2.5. Exposure to TCDD Increased Neuronal and TH-Positive Cell Populations
3. Discussion
4. Materials and Methods
4.1. Reagents and Plastic Wares
4.2. Feeder Cell Culture and Miscellaneous
4.3. Human ESC Maintenance Culture
4.4. O/L/F-Coated Plates
4.5. Neural Differentiation Culture
4.6. Quantitative RT-PCR Analysis
4.7. Transfection and Stably Transformed Cell Cloning
4.8. Immunocytochemistry and Image Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics
Abbreviations
| hESC | human embryonic stem cell |
| EST | embryonic stem cell test |
| EB | embryoid body |
| MEF | mouse embryonic fibroblast |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| AHR | aryl hydrocarbon receptor |
| MAP2 | microtubule-associated protein 2 |
| TH | thyroxine hydroxylase |
| ESM | embryonic stem cell medium |
| EBM | embryoid body medium |
| NIM | neuronal induction medium |
| NPM | neural proliferation medium |
| ROCK | Rho-associated coiled-coil forming kinase |
| O/L/F | ornithine/laminin/fibronectin |
| RAG | rat β-actin gene |
| EGFP | enhanced green fluorescent protein |
| DsRed | discosoma red fluorescent protein |
| IRES | internal ribosome entry site |
| ICC | immunocytochemistry |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| ANOVA | analysis of variance |
References
- Van den Berg, M.; Kypke, K.; Kotz, A.; Tritscher, A.; Lee, S.Y.; Magulova, K.; Fiedler, H.; Malisch, R. WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit–risk evaluation of breastfeeding. Arch. Toxicol. 2017, 91, 83–96. [Google Scholar] [CrossRef]
- Costopoulou, D.; Vassiliadou, I.; Chrysafidis, D.; Bergele, K.; Tzavara, E.; Tzamtzis, V.; Leondiadis, L. Determination of PCDD/F, dioxin-like PCB and PAH levels in olive and olive oil samples from areas affected by the fires in summer 2007 in Greece. Chemosphere 2010, 79, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hogaboam, J.P.; Moore, A.J.; Lawrence, B.P. The aryl hydrocarbon receptor affects distinct tissue compartments during ontogeny of the immune system. Toxicol. Sci. 2008, 102, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; DeWitt, J.C.; Germolec, D.R.; Zelikoff, J.T. Breaking Patterns of Environmentally Influenced Disease for Health Risk Reduction: Immune Perspectives. Environ. Health Perspect. 2010, 118, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Denison, M.S.; Birnbaum, L.S.; DeVito, M.J.; Fiedler, H.; Falandysz, J.; Rose, M.; Schrenk, D.; Safe, S.; Tohyama, C.; et al. Polybrominated Dibenzo-p-Dioxins, Dibenzofurans, and Biphenyls: Inclusion in the Toxicity Equivalency Factor Concept for Dioxin-Like Compounds. Toxicol. Sci. 2013, 133, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schantz, S.L.; Sweeney, A.M.; Gardiner, J.C.; Humphrey, H.E.B.; McCaffrey, R.J.; Gasior, D.M.; Srikanth, K.R.; Budd, M.L. Neuropsychological Assessment of an Aging Population of Great Lakes Fisheaters. Toxicol. Ind. Health 1996, 12, 403–417. [Google Scholar] [CrossRef]
- Bock, K.W. From TCDD-mediated toxicity to searches of physiologic AHR functions. Biochem. Pharmacol. 2018, 155, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, W.; Tohyama, C.; Yoshioka, W.; Tohyama, C. Mechanisms of Developmental Toxicity of Dioxins and Related Compounds. Int. J. Mol. Sci. 2019, 20, 617. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kakeyama, M.; Uemura, Y.; Haijima, A.; Okuno, H.; Bito, H.; Tohyama, C. Executive Function Deficits and Social-Behavioral Abnormality in Mice Exposed to a Low Dose of Dioxin In Utero and via Lactation. PLoS ONE 2012, 7, e50741. [Google Scholar] [CrossRef]
- Kakeyama, M.; Endo, T.; Zhang, Y.; Miyazaki, W.; Tohyama, C. Disruption of paired-associate learning in rat offspring perinatally exposed to dioxins. Arch. Toxicol. 2013, 88, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, E.; Ding, Y.; Tohyama, C. AhR signaling activation disrupts migration and dendritic growth of olfactory interneurons in the developing mouse. Sci. Rep. 2016, 6, 26386. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Kubo, K.; Endo, T.; Nakajima, K.; Kakeyama, M.; Tohyama, C. Excessive activation of AhR signaling disrupts neuronal migration in the hippocampal CA1 region in the developing mouse. J. Toxicol. Sci. 2017, 42, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Kimura, E.; Tohyama, C. Vocalization as a novel endpoint of atypical attachment behavior in 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed infant mice. Arch. Toxicol. 2018, 92, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Visek, W.J. Issues and current applications of interspecies extrapolation of carcinogenic potency as a component of risk assessment. Environ. Health Perspect. 1988, 77, 49–54. [Google Scholar] [CrossRef]
- Campbell, D.B. Extrapolation from animals to man. The integration of pharmacokinetics and pharmacodynamics. Ann. N. Y. Acad. Sci. 1996, 801, 116–135. [Google Scholar] [CrossRef]
- Gonzalez, F.J. Role of Gene Knockout and Transgenic Mice in the Study of Xenobiotic Metabolism. Drug Metab. Rev. 2003, 35, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.; Wilson, R. Predicting the carcinogenicity of chemicals in humans from rodent bioassay data. Environ. Health Perspect. 1991, 94, 195–218. [Google Scholar] [PubMed]
- Gomase, V.S.; Tagore, S. Species scaling and extrapolation. Curr. Drug Metab. 2008, 9, 193–198. [Google Scholar] [CrossRef]
- Brewster, D.W.; Matsumura, F. Differential effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipose tissue lipoprotein lipase activity in the guinea pig, rat, hamster, rabbit, and mink. Comp. Biochem. Physiol. C 1989, 93, 49–53. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.-Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Suemori, H.; Yasuchika, K.; Hasegawa, K.; Fujioka, T.; Tsuneyoshi, N.; Nakatsuji, N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem. Biophys. Res. Commun. 2006, 345, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Sasai, Y.; Eiraku, M.; Suga, H. In vitro organogenesis in three dimensions: Self-organising stem cells. Development 2012, 139, 4111–4121. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Toyoda, M.; Yamazaki-Inoue, M.; Fukawatase, Y.; Chikazawa, E.; Sakaguchi, H.; Akutsu, H.; Umezawa, A. DNA Methylation Dynamics in Human Induced Pluripotent Stem Cells over Time. PLoS Genet. 2011, 7, e1002085. [Google Scholar] [CrossRef]
- Stummann, T.C.; Bremer, S. The possible impact of human embryonic stem cells on safety pharmacological and toxicological assessments in drug discovery and drug development. Curr. Stem Cell Res. Ther. 2008, 3, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Stummann, T.C.; Hareng, L.; Bremer, S. Hazard assessment of methylmercury toxicity to neuronal induction in embryogenesis using human embryonic stem cells. Toxicology 2009, 257, 117–126. [Google Scholar] [CrossRef]
- He, X.; Imanishi, S.; Sone, H.; Nagano, R.; Qin, X.-Y.; Yoshinaga, J.; Akanuma, H.; Yamane, J.; Fujibuchi, W.; Ohsako, S. Effects of methylmercury exposure on neuronal differentiation of mouse and human embryonic stem cells. Toxicol. Lett. 2012, 212, 1–10. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Engber, T.M. Molecular neuroanatomic mechanisms of Parkinson’s disease: A proposed therapeutic approach. Neurol. Clin. 1992, 10, 435–449. [Google Scholar] [CrossRef]
- Kastner, A.; Hirsch, E.C.; Agid, Y.; Javoy-Agid, F. Tyrosine hydroxylase protein and messenger RNA in the dopaminergic nigral neurons of patients with Parkinson’s disease. Brain Res. 1993, 606, 341–345. [Google Scholar] [CrossRef]
- Min, N.; Joh, T.H.; Kim, K.S.; Peng, C.; Son, J.H. 5′ upstream DNA sequence of the rat tyrosine hydroxylase gene directs high-level and tissue-specific expression to catecholaminergic neurons in the central nervous system of transgenic mice. Brain Res. Mol. Brain Res. 1994, 27, 281–289. [Google Scholar] [CrossRef]
- Messam, C.A.; Hou, J.; Major, E.O. Coexpression of Nestin in Neural and Glial Cells in the Developing Human CNS Defined by a Human-Specific Anti-nestin Antibody. Exp. Neurol. 2000, 161, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Takei, Y.; Kikkawa, Y.S.; Atapour, N.; Hensch, T.K.; Hirokawa, N. Defects in Synaptic Plasticity, Reduced NMDA-Receptor Transport, and Instability of Postsynaptic Density Proteins in Mice Lacking Microtubule-Associated Protein 1A. J. Neurosci. 2015, 35, 15539–15554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwawaki, T.; Kohno, K.; Kobayashi, K. Identification of a Potential Nurr1 Response Element That Activates the Tyrosine Hydroxylase Gene Promoter in Cultured Cells. Biochem. Biophys. Res. Commun. 2000, 274, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Schepers, G.E.; Teasdale, R.D.; Koopman, P. Twenty pairs of sox: Extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev. Cell 2002, 3, 167–170. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Liu, R.; Zhang, K.; Zhang, Y. The lncRNA DEANR1 Facilitates Human Endoderm Differentiation by Activating FOXA2 Expression. Cell Rep. 2015, 11, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Cortés, F.; Debacker, C.; Péault, B.; Labastie, M.C. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech. Dev. 1999, 83, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Suemori, H.; Mizuseki, K.; Watanabe, K.; Urano, F.; Ichinose, H.; Haruta, M.; Takahashi, M.; Yoshikawa, K.; Nishikawa, S.-I.; et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl. Acad. Sci. USA 2002, 99, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.G.; Stice, S.S. Development and differentiation of neural rosettes derived from human embryonic stem cells. Stem Cell Rev. 2006, 2, 67–77. [Google Scholar] [CrossRef]
- Jiang, J.; Duan, Z.; Nie, X.; Xi, H.; Li, A.; Guo, A.; Wu, Q.; Jiang, S.; Zhao, J.; Chen, G. Activation of neuronal nitric oxide synthase (nNOS) signaling pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced neurotoxicity. Environ. Toxicol. Pharmacol. 2014, 38, 119–130. [Google Scholar] [CrossRef]
- Hays, L.E.; Carpenter, C.D.; Petersen, S.L. Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ. Health Perspect. 2002, 110 (Suppl. 3), 369–376. [Google Scholar] [CrossRef]
- Collins, L.L.; Williamson, M.A.; Thompson, B.D.; Dever, D.P.; Gasiewicz, T.A.; Opanashuk, L.A. 2,3,7,8-Tetracholorodibenzo-p-dioxin exposure disrupts granule neuron precursor maturation in the developing mouse cerebellum. Toxicol. Sci. 2008, 103, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Powers, B.E.; Lin, T.-M.; Vanka, A.; Peterson, R.E.; Juraska, J.M.; Schantz, S.L. Tetrachlorodibenzo-p-dioxin exposure alters radial arm maze performance and hippocampal morphology in female AhR mice. Genes Brain Behav. 2005, 4, 51–59. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Yonemoto, J.; Sone, H.; Kosuge, Y.; Kosaki, K.; Takahashi, T. In utero exposure to dioxin causes neocortical dysgenesis through the actions of p27Kip1. Proc. Natl. Acad. Sci. USA 2010, 107, 16331–16335. [Google Scholar] [CrossRef]
- Gassmann, K.; Abel, J.; Bothe, H.; Haarmann-Stemmann, T.; Merk, H.F.; Quasthoff, K.N.; Rockel, T.D.; Schreiber, T.; Fritsche, E. Species-Specific Differential AhR Expression Protects Human Neural Progenitor Cells against Developmental Neurotoxicity of PAHs. Environ. Health Perspect. 2010, 118, 1571–1577. [Google Scholar] [CrossRef] [Green Version]
- Latchney, S.E.; Lioy, D.T.; Henry, E.C.; Gasiewicz, T.A.; Strathmann, F.G.; Mayer-Pröschel, M.; Opanashuk, L.A. Neural precursor cell proliferation is disrupted through activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Stem Cells Dev. 2011, 20, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, E.; Yoshimura, S.; Uruno, S.; Ishihara-Sugano, M. Effect of dioxins on regulation of tyrosine hydroxylase gene expression by aryl hydrocarbon receptor: A neurotoxicology study. Environ. Heal. 2009, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, E.; Yoshimura, S.; Uruno, S.; Itoh, S.; Ishihara-Sugano, M. Tyrosine hydroxylase assay: A bioassay for aryl hydrocarbon receptor-active compounds based on tyrosine hydroxylase promoter activation. Toxicol. Mech. Methods 2012, 22, 458–460. [Google Scholar] [CrossRef]
- Tanida, T.; Tasaka, K.; Akahoshi, E.; Ishihara-Sugano, M.; Saito, M.; Kawata, S.; Danjo, M.; Tokumoto, J.; Mantani, Y.; Nagahara, D.; et al. Fetal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin transactivates aryl hydrocarbon receptor-responsive element III in the tyrosine hydroxylase immunoreactive neurons of the mouse midbrain. J. Appl. Toxicol. 2014, 34, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sethi, K.D. The Impact of Levodopa on Quality of Life in Patients With Parkinson Disease. Neurologist 2010, 16, 76–83. [Google Scholar] [CrossRef]
- Ogura, J.; Miyauchi, S.; Shimono, K.; Yang, S.; Gonchigar, S.; Ganapathy, V.; Bhutia, Y.D. Carbidopa is an activator of aryl hydrocarbon receptor with potential for cancer therapy. Biochem. J. 2017, 474, 3391–3402. [Google Scholar] [CrossRef] [PubMed]
- Wincent, E.; Bengtsson, J.; Bardbori, A.M.; Alsberg, T.; Luecke, S.; Rannug, U.; Rannug, A. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4479–4484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simunovic, M.; Brivanlou, A.H. Embryoids, organoids and gastruloids: New approaches to understanding embryogenesis. Development 2017, 144, 976–985. [Google Scholar] [CrossRef]
- Smith, R.C.; Tabar, V. Constructing and Deconstructing Cancers using Human Pluripotent Stem Cells and Organoids. Cell Stem Cell 2019, 24, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kugler, J.; Huhse, B.; Tralau, T.; Luch, A. Embryonic stem cells and the next generation of developmental toxicity testing. Expert Opin. Drug Metab. Toxicol. 2017, 13, 833–841. [Google Scholar] [CrossRef]
- Sawamoto, K.; Nakao, N.; Kobayashi, K.; Matsushita, N.; Takahashi, H.; Kakishita, K.; Yamamoto, A.; Yoshizaki, T.; Terashima, T.; Murakami, F.; et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 6423–6428. [Google Scholar] [CrossRef] [Green Version]
- Pannell, D.; Osborne, C.S.; Yao, S.; Sukonnik, T.; Pasceri, P.; Karaiskakis, A.; Okano, M.; Li, E.; Lipshitz, H.D.; Ellis, J. Retrovirus vector silencing is de novo methylase independent and marked by a repressive histone code. EMBO J. 2000, 19, 5884–5894. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, M.S.; Oses, C.; Vázquez Echegaray, C.; Solari, C.; Waisman, A.; Álvarez, Y.; Petrone, M.V.; Francia, M.; Schultz, M.; Sevlever, G.; et al. Kat6b Modulates Oct4 and Nanog Binding to Chromatin in Embryonic Stem Cells and Is Required for Efficient Neural Differentiation. J. Mol. Biol. 2019, 431, 1148–1159. [Google Scholar] [CrossRef]
- Muzzey, D.; van Oudenaarden, A. Quantitative Time-Lapse Fluorescence Microscopy in Single Cells. Annu. Rev. Cell Dev. Biol. 2009, 25, 301–327. [Google Scholar] [CrossRef] [Green Version]
- Ulloth, J.E.; Casiano, C.A.; De Leon, M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J. Neurochem. 2003, 84, 655–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.P.; Perez-Polo, J.R. Nerve growth factor induces neurite outgrowth in a clone derived from an NGF-insensitive human neuroblastoma cell line. Int. J. Dev. Neurosci. 1989, 7, 125–132. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Yoshioka, W.; Tohyama, C.; Ohsako, S. Internal genomic sequence of human CYP1A1 gene is involved in superinduction of dioxin-induced CYP1A1 transcription by cycloheximide. Biochem. Biophys. Res. Commun. 2007, 355, 687–692. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarma, S.N.; Nagano, R.; Ohsako, S. Tyroxine Hydroxylase-Positive Neuronal Cell Population is Increased by Temporal Dioxin Exposure at Early Stage of Differentiation from Human Embryonic Stem Cells. Int. J. Mol. Sci. 2019, 20, 2687. https://doi.org/10.3390/ijms20112687
Sarma SN, Nagano R, Ohsako S. Tyroxine Hydroxylase-Positive Neuronal Cell Population is Increased by Temporal Dioxin Exposure at Early Stage of Differentiation from Human Embryonic Stem Cells. International Journal of Molecular Sciences. 2019; 20(11):2687. https://doi.org/10.3390/ijms20112687
Chicago/Turabian StyleSarma, Sailendra Nath, Reiko Nagano, and Seiichiroh Ohsako. 2019. "Tyroxine Hydroxylase-Positive Neuronal Cell Population is Increased by Temporal Dioxin Exposure at Early Stage of Differentiation from Human Embryonic Stem Cells" International Journal of Molecular Sciences 20, no. 11: 2687. https://doi.org/10.3390/ijms20112687



