Improving Wettability: Deposition of TiO2 Nanoparticles on the O2 Plasma Activated Polypropylene Membrane
Abstract
:1. Introduction
2. Results and Discussion
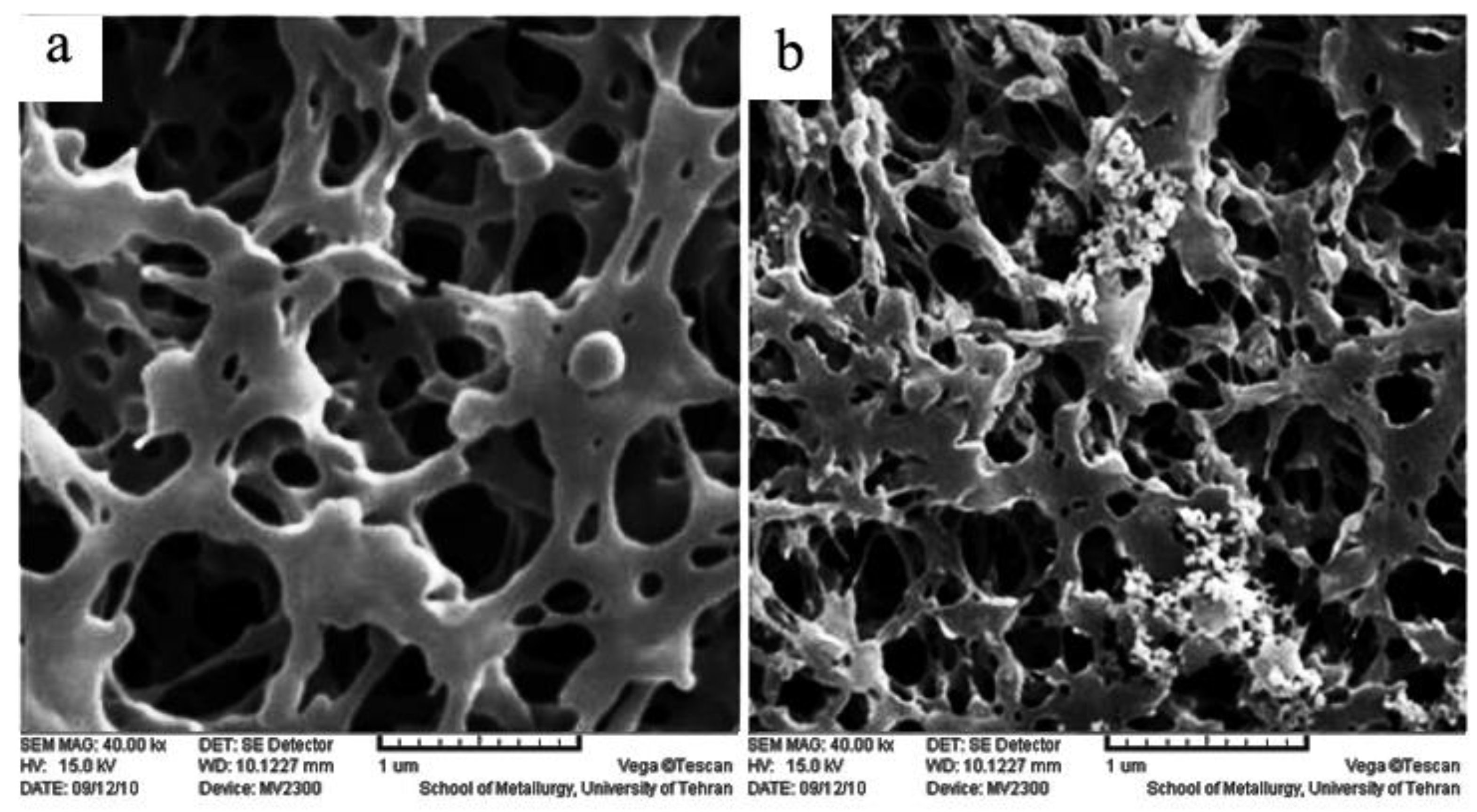
2.1. Scanning Electron Microscope (SEM)
2.2. X-Ray Diffraction (XRD)
2.3. Thermal Gravimetric Analysis (TGA)
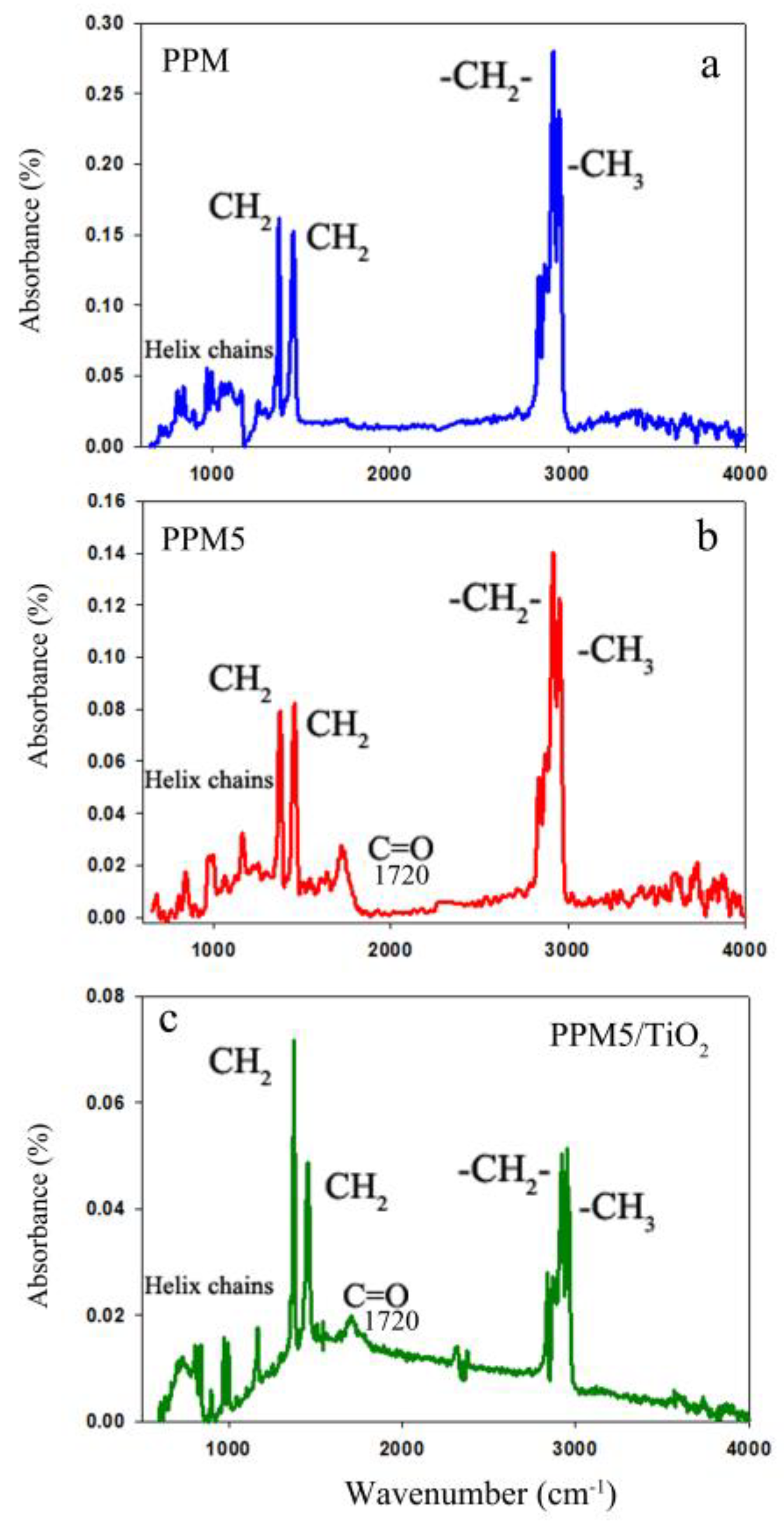
2.4. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
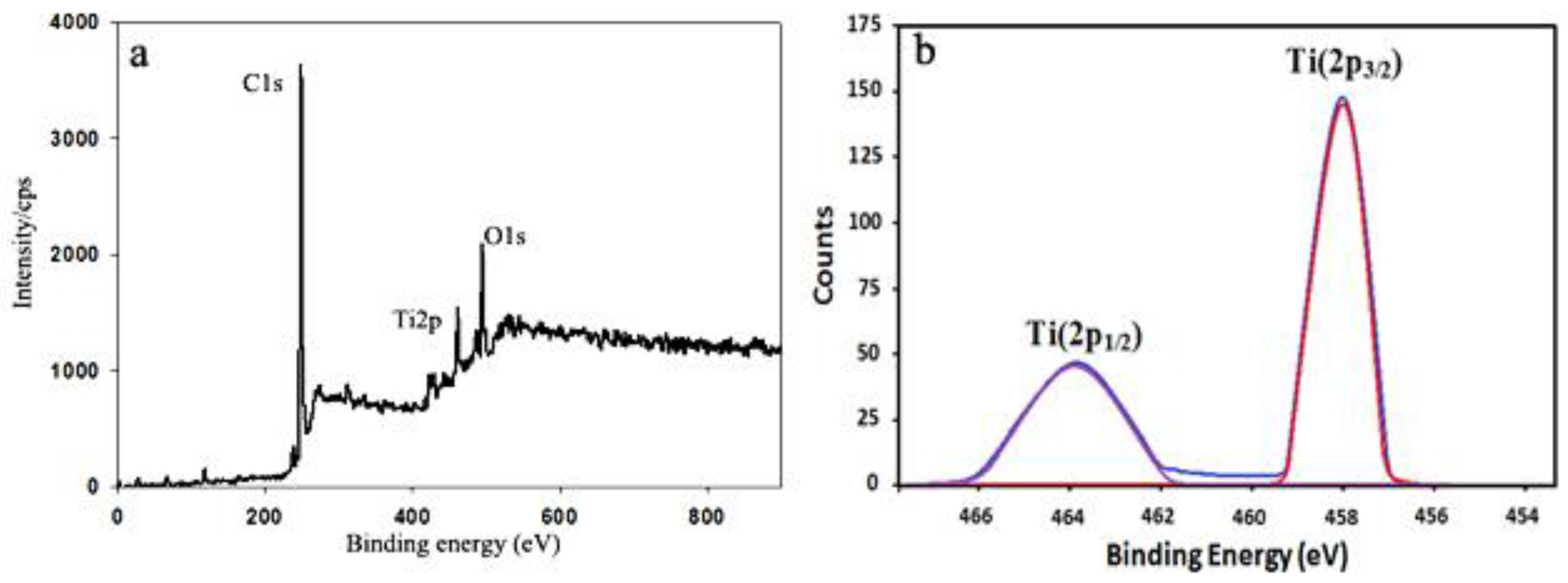
2.5. X-Ray Photoelectron Spectroscopy (XPS)
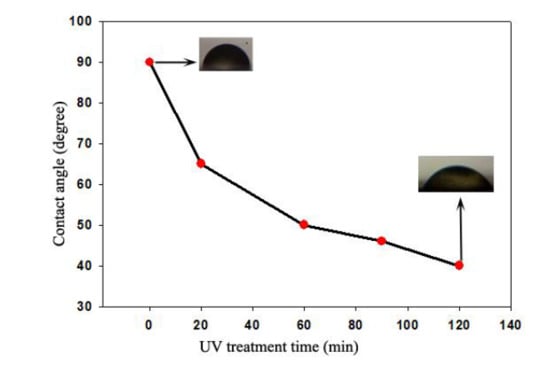
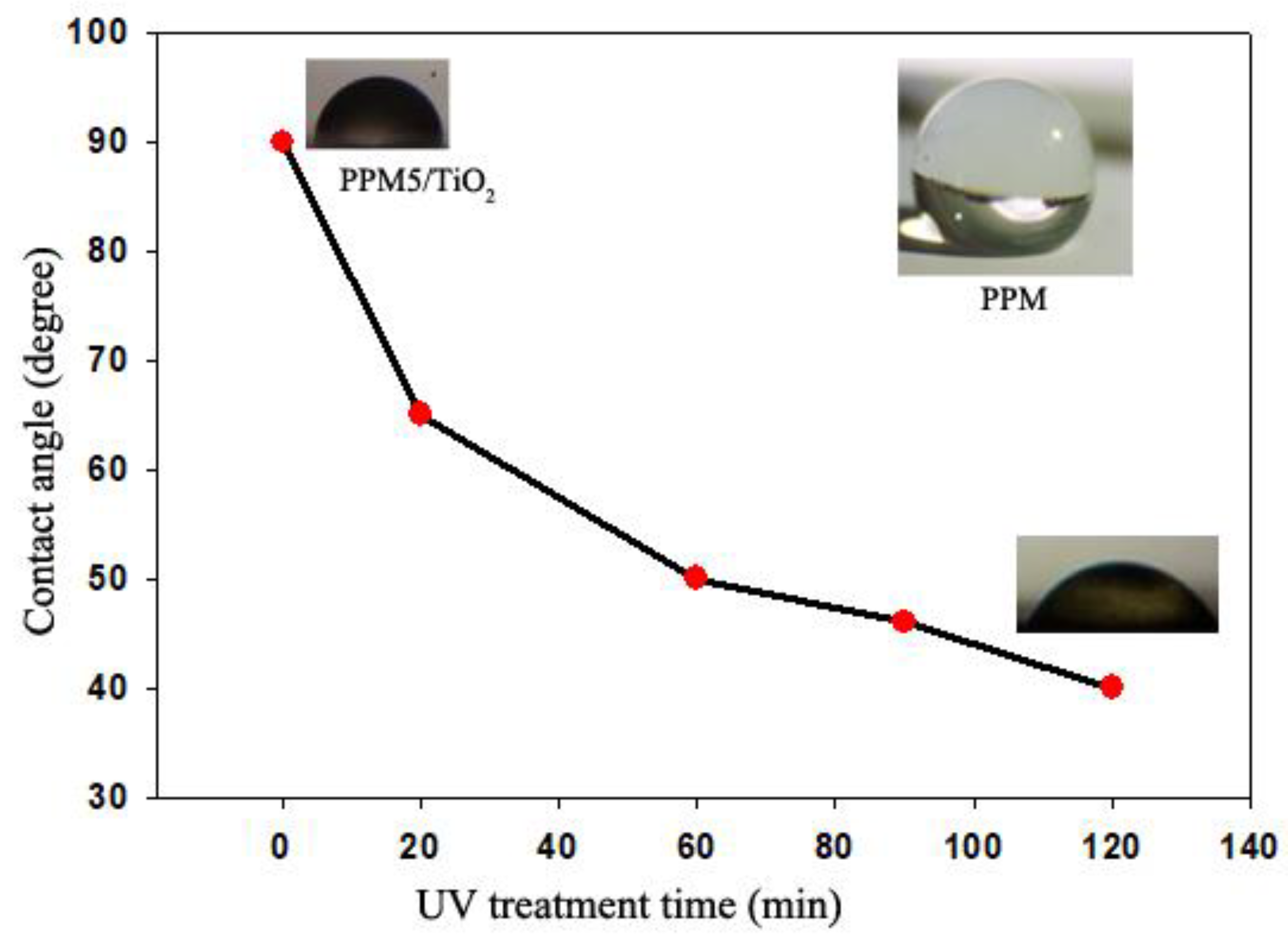
2.6. UV Treatment
2.7. Wettability
3. Materials and Methods
3.1. Preparation of Samples
3.2. Membrane Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, M.X.; Yang, Q.; Xu, Z.K. Enhancing the hydrophilicity of polypropylene microporous membranes by the grafting of 2-hydroxyethyl methacrylate via a synergistic effect of photoinitiators. J. Membr. Sci. 2006, 285, 196–205. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Z.K.; Dai, Z.W.; Wang, J.L.; Ulbricht, M. Surface modification of polypropylene microporous membranes with a novel glycopolymer. Chem. Mater. 2005, 17, 3050–3058. [Google Scholar] [CrossRef]
- Liang, L.; Feng, X.; Peurrung, L.; Viswanathan, V. Temperature-sensitive membranes prepared by UV photopolymerization of N-isopropylacrylamide on a surface of porous hydrophilic polypropylene membranes. J. Membr. Sci. 1999, 162, 235–246. [Google Scholar] [CrossRef]
- Qureshi, A.; Singh, D.; Singh, N.; Ataoglu, S.; Gulluoglu, A.N.; Tripathi, A.; Avasthi, D. Effect of irradiation by 140 Mev Ag11 + ions on the optical and electrical properties of polypropylene/TiO2 composite. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 3456–3460. [Google Scholar] [CrossRef]
- Li, C.; Yue, H.; Wang, Q.; Shi, M.; Zhang, H.; Li, X.; Dong, H.; Yang, S. A novel modified PP separator by grafting PAN for high-performance lithium–sulfur batteries. J. Mater. Sci. 2019, 54, 1566–1579. [Google Scholar] [CrossRef]
- Song, Y.Z.; Zhang, Y.; Yuan, J.J.; Lin, C.E.; Yin, X.; Sun, C.C.; Zhu, B.; Zhu, L.P. Fast assemble of polyphenol derived coatings on polypropylene separator for high performance lithium-ion batteries. J. Electroanal. Chem. 2018, 808, 252–258. [Google Scholar] [CrossRef]
- Tuominen, M.; Lahti, J.; Lavonen, J.; Penttinen, T.; Räsänen, J.P.; Kuusipalo, J. The influence of flame, corona and atmospheric plasma treatments on surface properties and digital print quality of extrusion coated paper. J. Adhes. Sci. Technol. 2010, 24, 471–492. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, J.F.; Ignacz, G.; Pogany, P.; Lee, Y.M.; Szekely, G. Bio-Inspired Robust Membranes Nanoengineered from Interpenetrating Polymer Networks of Polybenzimidazole/Polydopamine. ACS Nano 2019, 13, 125–133. [Google Scholar] [CrossRef]
- Yu, H.Y.; Tang, Z.Q.; Huang, L.; Cheng, G.; Li, W.; Zhou, J.; Yan, M.G.; Gu, J.S.; Wei, X.W. Surface modification of polypropylene macroporous membrane to improve its antifouling characteristics in a submerged membrane-bioreactor: H2O plasma treatment. Water Res. 2008, 42, 4341–4347. [Google Scholar] [CrossRef]
- Fisher, E.R. A Review of Plasma-Surface Interactions During Processing of Polymeric Materials Measured Using the IRIS Technique. Plasma Process. Polym. 2004, 1, 13–27. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B.; Xianda, S. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Jian, P.; Yahui, H.; Yang, W.; Linlin, L. Preparation of polysulfone-Fe3O4 composite ultrafiltration membrane and its behavior in magnetic field. J. Membr. Sci. 2006, 284, 9–16. [Google Scholar] [CrossRef]
- Chandramouleeswaran, S.; Mhaske, S.; Kathe, A.; Varadarajan, P.; Prasad, V.; Vigneshwaran, N. Functional behaviour of polypropylene/ZnO–soluble starch nanocomposites. Nanotechnology 2007, 18, 385702. [Google Scholar] [CrossRef]
- Altan, M.; Yildirim, H. Mechanical and antibacterial properties of injection molded polypropylene/TiO2 nano-composites: Effects of surface modification. J. Mater. Sci. Technol. 2012, 28, 686–692. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A. Preparation and characterization of novel porous PVDF-ZrO2 composite membranes. Desalination 2002, 146, 35–40. [Google Scholar] [CrossRef]
- Chin, S.S.; Chiang, K.; Fane, A.G. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Erdem, N.; Erdogan, U.H.; Cireli, A.A.; Onar, N. Structural and ultraviolet-protective properties of nano-TiO2-doped polypropylene filaments. J. Appl. Polym. Sci. 2010, 115, 152–157. [Google Scholar] [CrossRef]
- Luo, M.L.; Zhao, J.Q.; Tang, W.; Pu, C.S. Hydrophilic modification of poly (ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.; Taheri, A.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- Velásquez, J.; Valencia, S.; Rios, L.; Restrepo, G.; Marín, J. Characterization and photocatalytic evaluation of polypropylene and polyethylene pellets coated with P25 TiO2 using the controlled-temperature embedding method. Chem. Eng. J. 2012, 203, 398–405. [Google Scholar] [CrossRef]
- Yang, S.; Gu, J.S.; Yu, H.Y.; Zhou, J.; Li, S.F.; Wu, X.M.; Wang, L. Polypropylene membrane surface modification by RAFT grafting polymerization and TiO2 photocatalysts immobilization for phenol decomposition in a photocatalytic membrane reactor. Sep. Purif. Technol. 2011, 83, 157–165. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Bae, T.H.; Tak, T.M. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J. Membr. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Masaeli, E.; Morshed, M.; Tavanai, H. Study of the wettability properties of polypropylene nonwoven mats by low-pressure oxygen plasma treatment. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2007, 39, 770–774. [Google Scholar] [CrossRef]
- Szabová, R.; Černáková, L.; Wolfová, M.; Černák, M. Coating of TiO2 nanoparticles on the plasma activated polypropylene fibers. Acta Chim. Slovaca 2009, 2, 70–76. [Google Scholar]
- Chan, C.M.; Ko, T.M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Jaleh, B.; Shahbazi, N. Surface properties of UV irradiated PC–TiO2 nanocomposite film. Appl. Surf. Sci. 2014, 313, 251–258. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, J.; Dai, J.; Yang, Y.; Chen, X.; Wang, Y. Hydrophilization of porous polypropylene membranes by atomic layer deposition of TiO2 for simultaneously improved permeability and selectivity. J. Membr. Sci. 2013, 448, 215–222. [Google Scholar] [CrossRef]
- Chen, H.; Kong, L.; Wang, Y. Enhancing the hydrophilicity and water permeability of polypropylene membranes by nitric acid activation and metal oxide deposition. J. Membr. Sci. 2015, 487, 109–116. [Google Scholar] [CrossRef]
- Wang, S.; Ajji, A.; Guo, S.; Xiong, C. Preparation of microporous polypropylene/titanium dioxide composite membranes with enhanced electrolyte uptake capability via melt extruding and stretching. Polymers 2017, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aguirre, O.A.; Nunez-Pineda, A.; Tapia-Tapia, M.; Gomez Espinosa, R.M. Surface Modification of Polypropylene Membrane Using Biopolymers with Potential Applications for Metal Ion Removal. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Feizi Mohazzab, B.; Jaleh, B.; Kakuee, O.; Fattah-alhosseini, A. Formation of titanium carbide on the titanium surface using laser ablation in n-heptane and investigating its corrosion resistance. Appl. Surf. Sci. 2019, 478, 623–635. [Google Scholar] [CrossRef]
- Feizi Mohazzab, B.; Jaleh, B.; Nasrollahzadeh, M.; Issaabadi, Z. Journey on Greener Pathways via Synthesis of Pd/KB Polymeric Nanocomposite as a Recoverable Catalyst for the Ligand-Free Oxidative Hydroxylation of Phenylboronic Acid and Suzuki–Miyaura Coupling Reaction in Green Solvents. Catal. Lett. 2019, 149, 169–179. [Google Scholar] [CrossRef]
- Fonouni, M.; Yegani, R.; Tavakkoli, A.; Mollazadeh, S. Investigating the Effect of Various Oxidizing Agents on the Surface Functionalization of Microporous Polypropylene Membranes. J. Text. Polym. 2016, 4, 92–100. [Google Scholar]
- Jaleh, B.; Parvin, P.; Wanichapichart, P.; Saffar, A.P.; Reyhani, A. Induced super hydrophilicity due to surface modification of polypropylene membrane treated by O2 plasma. Appl. Surf. Sci. 2010, 257, 1655–1659. [Google Scholar] [CrossRef]
- Naghdi, S.; Jaleh, B.; Shahbazi, N. Reversible wettability conversion of electrodeposited graphene oxide/titania nanocomposite coating: Investigation of surface structures. Appl. Surf. Sci. 2016, 368, 409–416. [Google Scholar] [CrossRef]


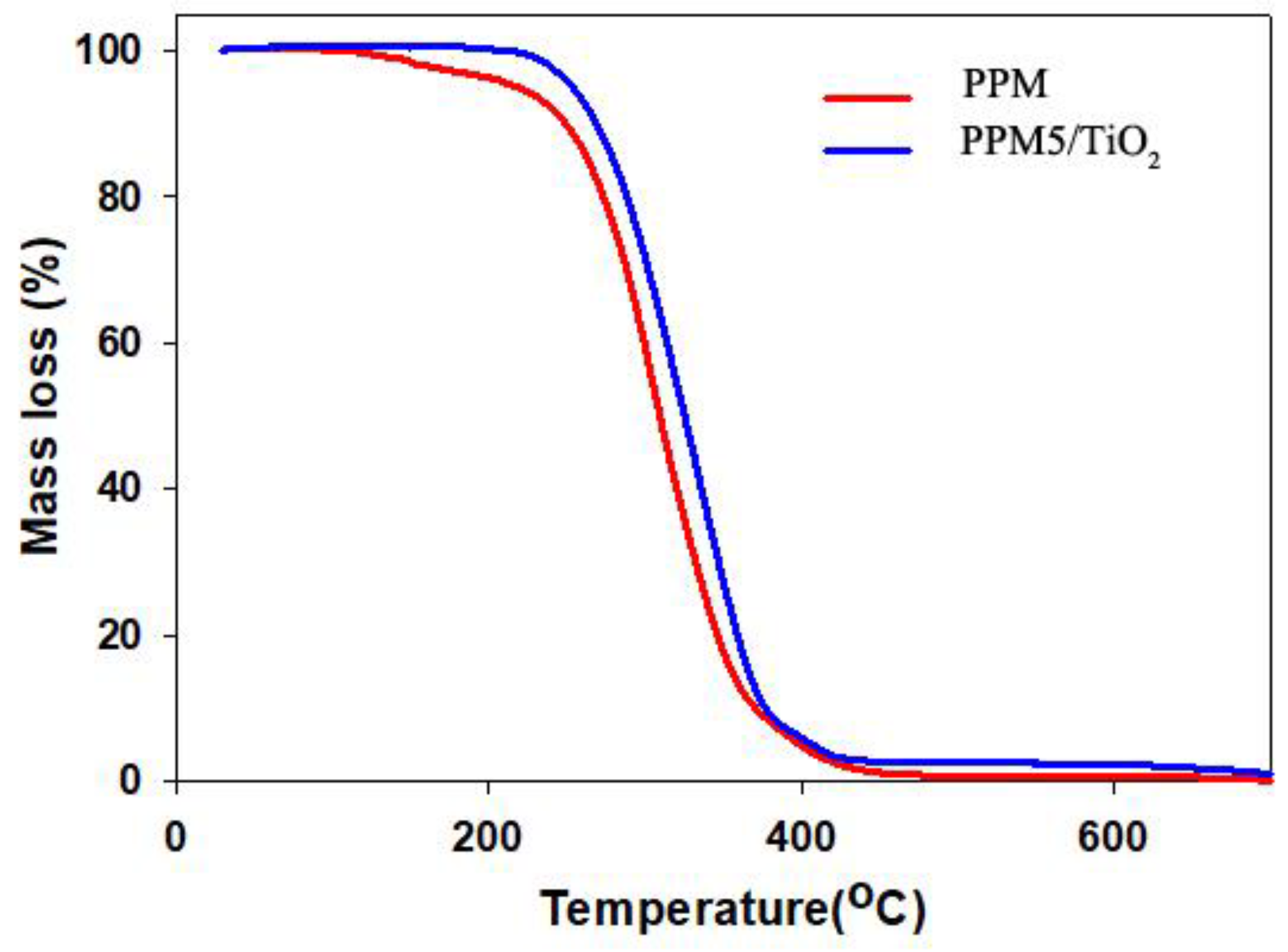
| Samples | Mass Loss Temperature (±2 °C) | ||
|---|---|---|---|
| TO | T50 | T90 | |
| PP membrane | 107 | 310 | 380 |
| PPM5/TiO2 | 213 | 358 | 394 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaleh, B.; Etivand, E.S.; Mohazzab, B.F.; Nasrollahzadeh, M.; Varma, R.S. Improving Wettability: Deposition of TiO2 Nanoparticles on the O2 Plasma Activated Polypropylene Membrane. Int. J. Mol. Sci. 2019, 20, 3309. https://doi.org/10.3390/ijms20133309
Jaleh B, Etivand ES, Mohazzab BF, Nasrollahzadeh M, Varma RS. Improving Wettability: Deposition of TiO2 Nanoparticles on the O2 Plasma Activated Polypropylene Membrane. International Journal of Molecular Sciences. 2019; 20(13):3309. https://doi.org/10.3390/ijms20133309
Chicago/Turabian StyleJaleh, Babak, Ehsan Sabzi Etivand, Bahareh Feizi Mohazzab, Mahmoud Nasrollahzadeh, and Rajender S. Varma. 2019. "Improving Wettability: Deposition of TiO2 Nanoparticles on the O2 Plasma Activated Polypropylene Membrane" International Journal of Molecular Sciences 20, no. 13: 3309. https://doi.org/10.3390/ijms20133309