Cytosolic Copper Binding by a Bacterial Storage Protein and Interplay with Copper Efflux
Abstract
1. Introduction
2. Results
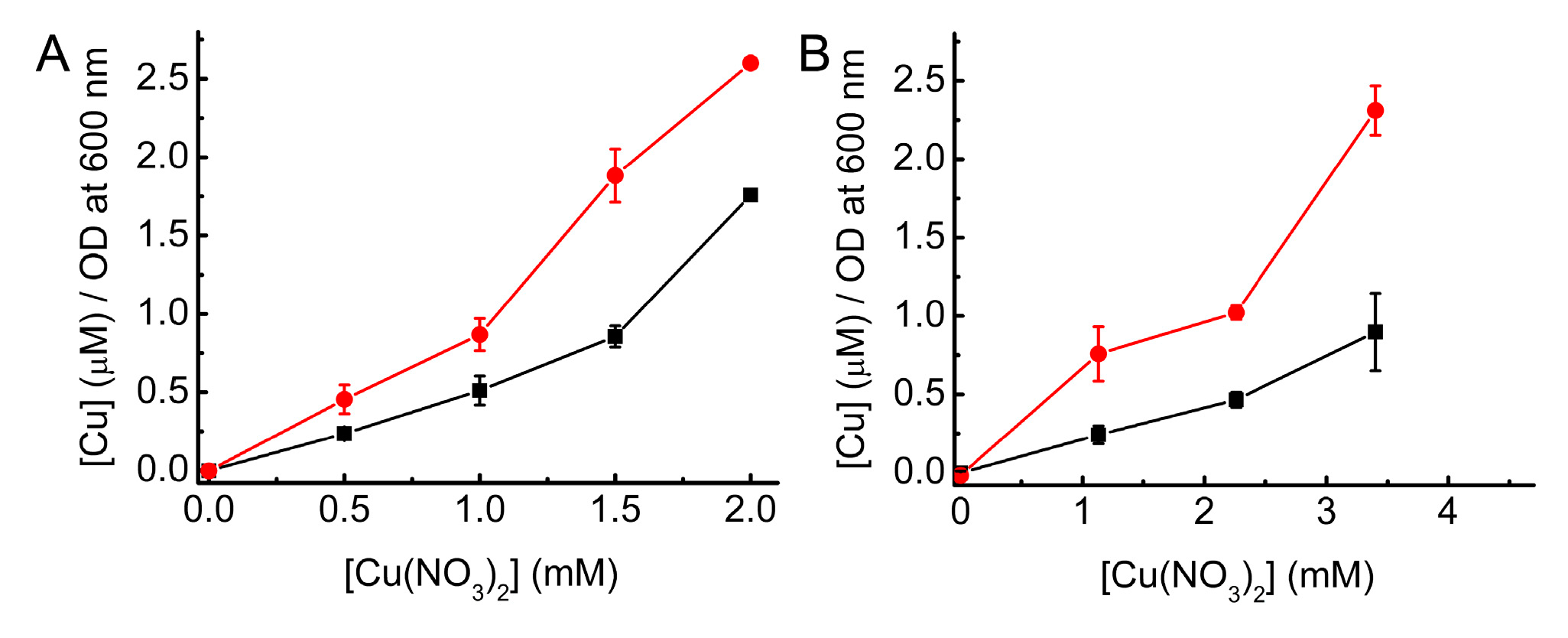
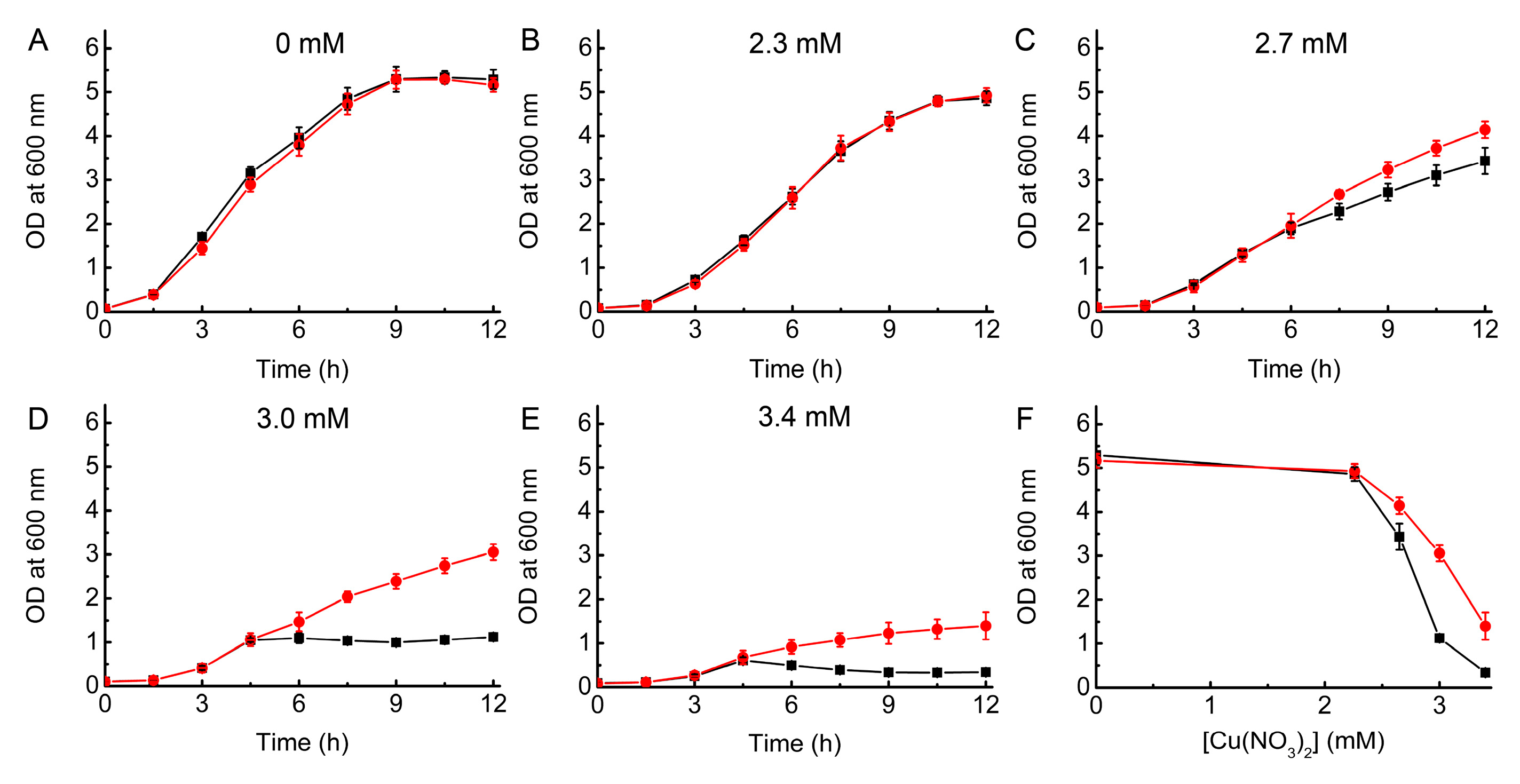
2.1. Growth of WT and ΔcopA E. coli as a Function of Cu Concentration
2.2. Complementation Studies of ΔcopA E. coli
2.3. Testing the Ability of BsCsp3 to Confer Resistance to Cu Toxicity in E. coli
2.4. Testing the Ability of MtCsp3 to Confer Resistance to Cu Toxicity in E. coli
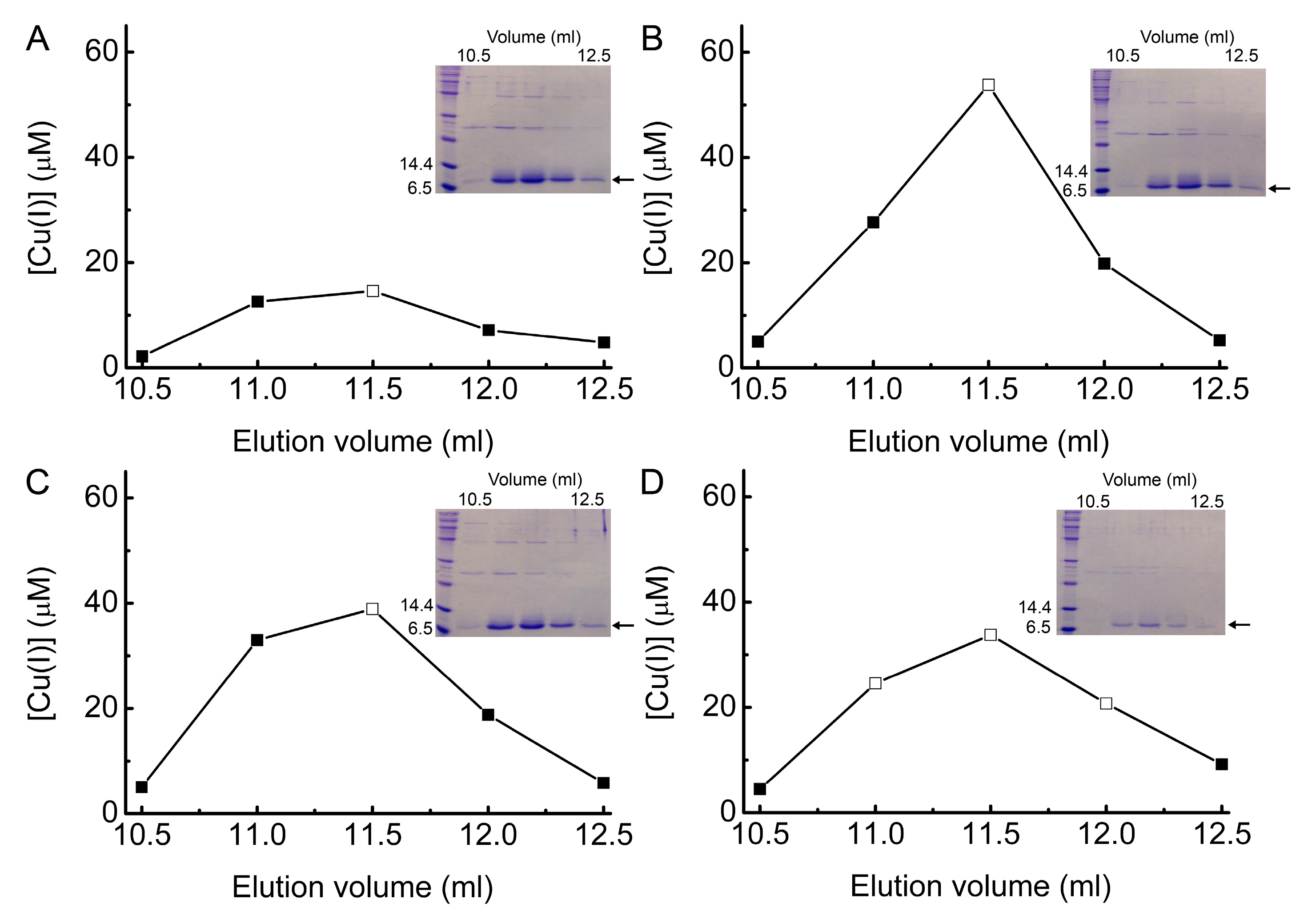
2.5. Investigating Intracellular Cu Binding by BsCsp3 in E. coli
3. Discussion
4. Materials and Methods
4.1. Analysis of WT and the copA Deletion Strains of E. coli BW25113 and the Influence of BsCsp3 Overexpression
4.2. Purification and Cu(I)-Binding Stoichiometry of BsCsp3
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vita, N.; Platsaki, S.; Baslé, A.; Allen, S.J.; Paterson, N.G.; Crombie, A.T.; Murrell, J.C.; Waldron, K.J.; Dennison, C. A four-helix bundle stores copper for methane oxidation. Nature 2015, 525, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Vita, N.; Landolfi, G.; Baslé, A.; Platsaki, S.; Lee, J.; Waldron, K.J.; Dennison, C. Bacterial cytosolic proteins with a high capacity for Cu(I) that protect against copper toxicity. Sci. Rep. 2016, 6, 39065. [Google Scholar] [CrossRef] [PubMed]
- Baslé, A.; Platsaki, S.; Dennison, C. Visualizing biological copper storage: The importance of thiolate-coordinated tetranuclear clusters. Angew. Chem. Int. Ed. 2017, 56, 8697–8700. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.; David, S.; Lee, J. Bacterial copper storage proteins. J. Biol. Chem. 2018, 293, 4616–4627. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C. The coordination chemistry of copper uptake and storage for methane oxidation. Chem. Eur. J. 2019, 25, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.; Berks, B.C. The twin-arginine translocation (Tat) protein export pathway. Nat. Rev. Microbiol. 2012, 10, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Semrau, J.D. Copper and cerium-regulated gene expression in Methylosinus trichosporium OB3b. Appl. Microbiol. Biotechnol. 2017, 101, 8499–8516. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper toxicity and the origin of bacterial resistance-new insights and applications. Metallomics 2011, 3, 1109–1118. [Google Scholar] [CrossRef]
- Solioz, M. Copper toxicity. In Copper and Bacteria—Evolution, Homeostasis and Toxicity; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Fung, D.K.C.; Lau, W.Y.; Chan, W.T.; Yan, A. Copper efflux is induced during anaerobic amino acid limitation in Escherichia coli to protect iron-sulfur cluster enzymes and biogenesis. J. Bacteriol. 2013, 195, 4556–4568. [Google Scholar] [CrossRef]
- Chillappagari, S.; Seubert, A.; Trip, H.; Kruipers, O.P.; Marahiel, M.A.; Miethke, M. Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis. J. Bacteriol. 2010, 192, 2512–2524. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Cheng, Z.; Pang, Y.; Landry, A.P.; Li, J.; Ding, H. Copper binding in IscA inhibits iron-sulfur cluster assembly in Escherichia coli. Mol. Microbiol. 2014, 93, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Changela, A.; Chen, K.; Xue, Y.; Holschen, J.; Outten, C.E.; O’Halloran, T.V.; Mondragón, A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 2003, 301, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Rensing, C.; McDevitt, S.F. The copper metallome in prokaryotic cells. Met. Ions Life Sci. 2013, 12, 417–450. [Google Scholar] [PubMed]
- Tottey, S.; Waldron, K.J.; Firbank, S.J.; Reale, B.; Bessant, C.; Sato, K.; Cheek, T.R.; Gray, J.; Banfield, M.J.; Dennison, C.; et al. Protein-folding location can regulate manganese-binding versus copper-or zinc-binding. Nature 2008, 455, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Møller, L.B. Control of copper homeostasis in Escherichia coli by a P-type ATPase, CopA, and a MerR-like transcriptional activator, CopR. Gene 2000, 261, 289–298. [Google Scholar] [CrossRef]
- Rensing, C.; Fan, B.; Sharma, R.; Mitra, B.; Rosen, B.P. CopA: An Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 2000, 97, 652–656. [Google Scholar] [CrossRef]
- Fan, B.; Grass, G.; Rensing, C.; Rosen, B.P. Escherichia coli CopA N-terminal Cys(X)2Cys motifs are not required for copper resistance or transport. Biochem. Biophys. Res. Commun. 2001, 286, 414–418. [Google Scholar] [CrossRef]
- Fan, B.; Rosen, B.P. Biochemical characterization of CopA, the Escherichia coli Cu(I)-translocating P-type ATPase. J. Biol. Chem. 2002, 277, 46987–46992. [Google Scholar] [CrossRef]
- Rensing, C.; Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 2003, 27, 197–213. [Google Scholar] [CrossRef]
- Solioz, M.; Abicht, H.K.; Mermod, M.; Mancini, S. Response of gram-positive bacteria to copper stress. J. Biol. Inorg. Chem. 2010, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gourdon, P.; Liu, X.; Skjørringe, T.; Morth, J.P.; Møller, L.B.; Pedersen, B.P.; Nissen, P. Crystal structure of a copper-transporting PIB-type ATPase. Nature 2011, 475, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Argüello, J.M.; Raimunda, D.; Padilla-Benavides, T. Mechanisms of copper homeostasis in bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Drees, S.L.; Beyer, D.F.; Lenders-Lomscher, C.; Lübben, M. Distinct functions of serial metal-binding domains in the Escherichia coli P1B-ATPase CopA. Mol. Microbiol. 2015, 97, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Meydan, S.; Klepacki, D.; Karthikeyan, S.; Margus, T.; Thomas, P.; Jones, J.E.; Khan, Y.; Briggs, J.; Dinman, J.D.; Vázquez-Laslop, N.; et al. Programmed ribosomal frameshifting generates a copper transporter and a copper chaperone from the same gene. Mol. Cell 2017, 65, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.; Wickramasinghe, W.A.; Harrison, M.D.; Weber, T.; Solioz, M.; Dameron, C.T. The Enterococcus hirae copper chaperone CopZ delivers copper(I) to the CopY repressor. FEBS Lett. 1999, 445, 27–30. [Google Scholar] [CrossRef]
- Multhaup, G.; Strausak, D.; Bissig, K.D.; Solioz, M. Interaction of the CopZ copper chaperone with the CopA copper ATPase of Enterococcus hirae assessed by surface plasmon resonance. Biochem. Biophys. Res. Commun. 2001, 288, 172–177. [Google Scholar] [CrossRef]
- Outten, F.W.; Outten, C.E.; Hale, J.A.; O’Halloran, T.V. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, CueR. J. Biol. Chem. 2000, 275, 31024–31029. [Google Scholar] [CrossRef]
- Smaldone, G.T.; Helmann, J.D. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology 2007, 153, 4123–4128. [Google Scholar] [CrossRef]
- Liu, T.; Ramesh, A.; Ma, Z.; Ward, S.K.; Zhang, L.; George, G.N.; Talaat, A.M.; Sacchettini, J.C.; Giedroc, D.P. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat. Chem. Biol. 2007, 3, 60–68. [Google Scholar] [CrossRef]
- Odermatt, A.; Solioz, M. Two trans-acting metalloregulatory proteins controlling expression of the copper-ATPases of Enterococcus hirae. J. Biol. Chem. 1995, 270, 4349–4354. [Google Scholar] [CrossRef] [PubMed]
- Strausak, D.; Solioz, M. CopY is a copper-inducible repressor of the Enterococcus hirae copper ATPases. J. Biol. Chem. 1997, 272, 8932–8986. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Grass, G.; Rensing, C.; Montfort, W.R. Cuprous oxidase activity of CueO from Escherichia coli. J. Bacteriol. 2004, 186, 7815–7817. [Google Scholar] [CrossRef] [PubMed]
- Dwarakanath, S.; Chaplin, A.K.; Hough, M.A.; Rigali, S.; Vijgenboom, E.; Worrall, J.A. Response to copper stress in Streptomyces lividans extends beyond genes under direct control of a copper-sensitive operon repressor protein (CsoR). J. Biol. Chem. 2012, 287, 17833–17847. [Google Scholar] [CrossRef] [PubMed]
- Straw, M.L.; Chaplin, A.K.; Hough, M.A.; Paps, J.; Bavro, V.N.; Wilson, M.T.; Vijgenboom, E.; Worrall, J.A.R. A cytosolic copper storage protein provides a second level of copper tolerance in Streptomyces lividans. Metallomics 2018, 10, 180–193. [Google Scholar] [CrossRef]
- González-Quiñónez, N.; Corte-Rodríguez, M.; Álvarez-Fernández-García, R.; Rioseras, B.; López-García, M.T.; Fernández-García, G.; Montes-Bayón, M.; Manteca, A.; Yagüe, P. Cytosolic copper is a major modulator of germination, development and secondary metabolism in Streptomyces coelicolor. Sci. Rep. 2019, 9, 4214. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Novoa-Aponte, L.; Argüello, J.M. Copper homeostasis networks in the bacterium Pseudomonas aeruginosa. J. Biol. Chem. 2017, 292, 15691–15704. [Google Scholar] [CrossRef]
- Stolle, P.; Hou, B.; Brüser, T. The Tat substrate CueO is transported in an incomplete folding state. J. Biol. Chem. 2016, 291, 13520–13528. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Raimunda, D.; Cheng, X.; Argüello, J.M. Distinct functional roles of homologous Cu+ efflux ATPases in Pseudomonas aeruginosa. Mol. Microbiol. 2010, 78, 1246–1258. [Google Scholar] [CrossRef]
- Helbig, K.; Bleuel, C.; Krauss, G.J.; Nies, D.H. Glutathione and transition-metal homeostasis in Escherichia coli. J. Bacteriol. 2008, 190, 5431–5438. [Google Scholar] [CrossRef]
- Bowers, L.M.; Lapoint, K.; Anthony, L.; Pluciennik, A.; Filutowicz, M. Bacterial expression system with tightly regulated gene expression and plasmid copy number. Gene 2004, 340, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Young, C.A.; Gordon, L.D.; Fang, Z.; Holder, R.C.; Reid, S.D. Copper tolerance and characterization of a copper-responsive operon, copYAZ, in an M1T1 clinical strain of Streptococcus pyogenes. J. Bacteriol. 2015, 197, 2580–2592. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.V.; Muzyka, N.G.; Glukhovchenko, N.; Oktyabrsky, O.N. Effects of menadione and hydrogen peroxide on glutathione status in growing Escherichia coli. Free Radic. Biol. Med. 2000, 28, 1009–1016. [Google Scholar] [CrossRef]
- Newton, G.L.; Rawat, M.; La Clair, J.J.; Jothivasan, V.K.; Budiarto, T.; Hamilton, C.J.; Claiborne, A.; Helmann, J.D.; Fahey, R.C. Bacillithiol is an antioxidant thiol produced in Bacilli. Nat. Chem. Biol. 2009, 5, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Hguyen, L.A.H.; Hancock, H.L.; Fahrni, C.J. Glutathione limits aquacopper(I) to sub-femtomolar concentrations through cooperative assembly of a tetranuclear cluster. J. Biol. Chem. 2017, 292, 21558–21567. [Google Scholar] [CrossRef] [PubMed]
- Hoegler, K.J.; Hecht, M.H. A de novo protein confers copper resistance in Escherichia coli. Protein Sci. 2016, 25, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 1–11. [Google Scholar] [CrossRef]
- Badarau, A.; Firbank, S.J.; McCarthy, A.A.; Banfield, M.J.; Dennison, C. Visualizing the metal-binding versatility of copper trafficking sites. Biochemistry 2010, 49, 7798–7810. [Google Scholar] [CrossRef] [PubMed]
- Badarau, A.; Dennison, C. Copper trafficking mechanism of CXXC-containing domains: Insight from the pH-dependence of their Cu(I) affinities. J. Am. Chem. Soc. 2011, 133, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Badarau, A.; Dennison, C. Cu(I) affinities of the domain 1 and 3 sites in the human metallochaperone for Cu, Zn-superoxide dismutase. Biochemistry 2012, 51, 1439–1448. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity gradients drive copper to cellular destinations. Nature 2010, 465, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Badarau, A.; Dennison, C. Thermodynamics of copper and zinc distribution in the cyanobacterium Synechocystis PCC 6803. Proc. Natl. Acad. Sci. USA 2011, 108, 13007–13012. [Google Scholar] [CrossRef] [PubMed]




| E. coli Strain and Added Cu(NO3)2 Concentration | [Cu(I)] (µM) | [BsCsp3] (µM) | [Cu(I)]/[BsCsp3] 2 |
|---|---|---|---|
| ΔcopA in 1.0 mM Cu(NO3)2 | 50.9 | 45.7 | 1.1 |
| ΔcopA in 1.5 mM Cu(NO3)2 | 162 | 39.1 | 4.1 |
| WT in 1.5 mM Cu(NO3)2 | 166 | 38.7 | 4.3 |
| WT in 3.4 mM Cu(NO3)2 | 189 | 20.2 | 9.4 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Dennison, C. Cytosolic Copper Binding by a Bacterial Storage Protein and Interplay with Copper Efflux. Int. J. Mol. Sci. 2019, 20, 4144. https://doi.org/10.3390/ijms20174144
Lee J, Dennison C. Cytosolic Copper Binding by a Bacterial Storage Protein and Interplay with Copper Efflux. International Journal of Molecular Sciences. 2019; 20(17):4144. https://doi.org/10.3390/ijms20174144
Chicago/Turabian StyleLee, Jaeick, and Christopher Dennison. 2019. "Cytosolic Copper Binding by a Bacterial Storage Protein and Interplay with Copper Efflux" International Journal of Molecular Sciences 20, no. 17: 4144. https://doi.org/10.3390/ijms20174144
APA StyleLee, J., & Dennison, C. (2019). Cytosolic Copper Binding by a Bacterial Storage Protein and Interplay with Copper Efflux. International Journal of Molecular Sciences, 20(17), 4144. https://doi.org/10.3390/ijms20174144





