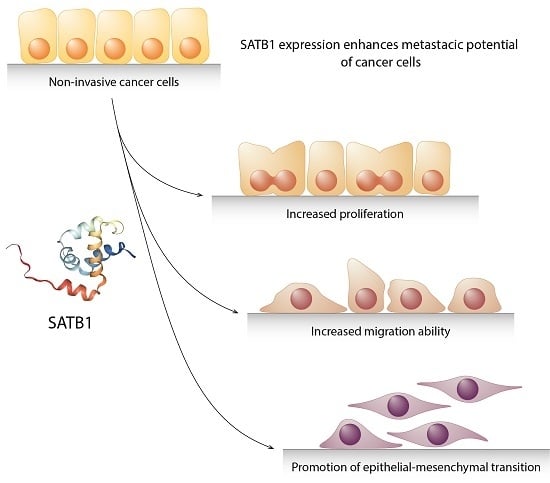
The Role of SATB1 in Tumour Progression and Metastasis
Abstract
:1. Introduction
SATB1 Protein
2. SATB1’s Role in Cancer Progression
2.1. Breast Cancer
2.2. Lung Cancer
2.3. Colorectal Cancer
2.4. Prostate Cancer
2.5. Gastric Cancer
2.6. Other Neoplasms
3. Conclusions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M. Causes of Death. Available online: https://ourworldindata.org/causes-of-death (accessed on 13 July 2018).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- American Institute of Cancer Research Cancer Trends. Comparing More and Less Developed Countries. Available online: https://www.wcrf.org/int/cancer-trends/comparing-more-less-developed-countries (accessed on 13 July 2018).
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited-the role of tumor-stroma interactions in metastasis to different organs. Int. J. cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Ewing, J. Neoplastic Diseases: A Treatise on Tumors; W. B. Saunders Company: Philadelphia, PA, USA; London, UK, 1919. [Google Scholar]
- Li, W.; Kang, Y. Probing the Fifty Shades of EMT in Metastasis. Trends in cancer 2016, 2, 65–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diepenbruck, M.; Christofori, G. Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef]
- Li, C.; Balazsi, G. A landscape view on the interplay between EMT and cancer metastasis. Syst. Biol. Appl. 2018, 4, 34. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, X.; Xu, G.; Liu, S. Metastasis: an early event in cancer progression. J. Cancer Res. Clin. Oncol. 2017, 143, 745–757. [Google Scholar] [CrossRef]
- Nordling, C.O. A new theory on cancer-inducing mechanism. Br. J. Cancer 1953, 7, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Doll, R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer 1954, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Nowak, M.A.; Marchionni, L.; Vogelstein, B.; Parmigiani, G. Only three driver gene mutations are required for the development of lung and colorectal cancers. Proc. Natl. Acad. Sci. USA 2014, 112, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.-J.; Russo, J.; Kohwi, Y.; Kohwi-Shigematsu, T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature 2008, 452, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.A.; Joh, T.; Kohwi, Y.; Kohwi-Shigematsu, T. A tissue-specific MARSAR DNA-binding protein with unusual binding site recognition. Cell 1992, 70, 631–645. [Google Scholar] [CrossRef]
- Kohwi-Shigematsu, T.; Kohwi, Y.; Takahashi, K.; Richards, H.W.; Ayers, S.D.; Han, H.-J.; Cai, S. SATB1-mediated functional packaging of chromatin into loops. Methods 2012, 58, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galande, S.; Purbey, P.K.; Notani, D.; Kumar, P.P. The third dimension of gene regulation: organization of dynamic chromatin loopscape by SATB1. Curr. Opin. Genet. Dev. 2007, 17, 408–414. [Google Scholar] [CrossRef]
- Kohwi-Shigematsu, T.; Poterlowicz, K.; Ordinario, E.; Han, H.-J.; Botchkarev, V.A.; Kohwi, Y. Genome organizing function of SATB1 in tumor progression. Semin. Cancer Biol. 2013, 23, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Pavan Kumar, P.; Purbey, P.K.; Sinha, C.K.; Notani, D.; Limaye, A.; Jayani, R.S.; Galande, S. Phosphorylation of SATB1, a global gene regulator, acts as a molecular switch regulating its transcriptional activity in vivo. Mol. Cell 2006, 22, 231–243. [Google Scholar] [CrossRef]
- Purbey, P.K.; Singh, S.; Notani, D.; Kumar, P.P.; Limaye, A.S.; Galande, S. Acetylation-dependent interaction of SATB1 and CtBP1 mediates transcriptional repression by SATB1. Mol. Cell. Biol. 2009, 29, 1321–1337. [Google Scholar] [CrossRef]
- Nagpal, N.; Ahmad, H.M.; Molparia, B.; Kulshreshtha, R. MicroRNA-191, an estrogen-responsive microRNA, functions as an oncogenic regulator in human breast cancer. Carcinogenesis 2013, 34, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.Y.; Dayan, S.; Wong, S.W.; Kaczmarek, A.; Hope, C.M.; Pederson, S.M.; Arnet, V.; Goodall, G.J.; Russell, D.; Sadlon, T.J.; et al. FOXP3 and miR-155 cooperate to control the invasive potential of human breast cancer cells by down regulating ZEB2 independently of ZEB1. Oncotarget 2018, 9, 27708–27727. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Q.; Chen, Z.-Q.; Cao, X.-X.; Xu, J.-D.; Xu, J.-W.; Chen, Y.-Y.; Wang, W.-J.; Chen, Q.; Tang, F.; Liu, X.-P.; et al. Involvement of NF-κB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ. 2011, 18, 16–25. [Google Scholar] [CrossRef] [PubMed]
- McInnes, N.; Sadlon, T.J.; Brown, C.Y.; Pederson, S.; Beyer, M.; Schultze, J.L.; McColl, S.; Goodall, G.J.; Barry, S.C. FOXP3 and FOXP3-regulated microRNAs suppress SATB1 in breast cancer cells. Oncogene 2012, 31, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, D.; Huo, J.; Kong, F.; Yang, H.; Ma, X. LINC01016 promotes the malignant phenotype of endometrial cancer cells by regulating the miR-302a-3p/miR-3130-3p/NFYA/SATB1 axis. Cell Death Dis. 2018, 9, 303. [Google Scholar] [CrossRef]
- Lopes-Ramos, C.M.; Habr-Gama, A.; Quevedo, B.; de, S.; Felício, N.M.; Bettoni, F.; Koyama, F.C.; Asprino, P.F.; Galante, P.A.; Gama-Rodrigues, J.; et al. Overexpression of miR-21-5p as a predictive marker for complete tumor regression to neoadjuvant chemoradiotherapy in rectal cancer patients. BMC Med. Genomics 2014, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.E.; Krazinski, B.E.; Godlewski, J.; Grzegrzolka, J.; Kiewisz, J.; Kwiatkowski, P.; Sliwinska-Jewsiewicka, A.; Dziegiel, P.; Kmiec, Z. SATB1 is Down-regulated in Clear Cell Renal Cell Carcinoma and Correlates with miR-21-5p Overexpression and Poor Prognosis. Cancer Genomics Proteomics 2016, 13, 209–217. [Google Scholar]
- Fessing, M.Y.; Mardaryev, A.N.; Gdula, M.R.; Sharov, A.A.; Sharova, T.Y.; Rapisarda, V.; Gordon, K.B.; Smorodchenko, A.D.; Poterlowicz, K.; Ferone, G.; et al. p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. J. Cell Biol. 2011, 194, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.D.; Yasui, D.H.; Niida, H.; Joh, T.; Loh, D.Y.; Kohwi-Shigematsu, T. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000, 14, 521–535. [Google Scholar]
- Savarese, F.; Dávila, A.; Nechanitzky, R.; De La Rosa-Velazquez, I.; Pereira, C.F.; Engelke, R.; Takahashi, K.; Jenuwein, T.; Kohwi-Shigematsu, T.; Fisher, A.G.; et al. Satb1 and Satb2 regulate embryonic stem cell differentiation and Nanog expression. Genes Dev. 2009, 23, 2625–2638. [Google Scholar] [CrossRef]
- Baguma-Nibasheka, M.; Angka, H.E.; Inanlou, M.R.; Kablar, B. Microarray analysis of Myf5-/-:MyoD-/- hypoplastic mouse lungs reveals a profile of genes involved in pneumocyte differentiation. Histol. Histopathol. 2007, 22, 483–495. [Google Scholar] [PubMed]
- Zhang, H.; Qu, S.; Li, S.; Wang, Y.; Li, Y.; Wang, Y.; Wang, Z.; Li, R. Silencing SATB1 inhibits proliferation of human osteosarcoma U2OS cells. Mol. Cell. Biochem. 2013, 378, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-H.; Ma, Y.-B.; Feng, D.-F.; Zhang, H.; Zhu, Z.-A.; Li, Z.-Q.; Jiang, P.-C. Upregulation of SATB1 is associated with the development and progression of glioma. J. Transl. Med. 2012, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Frömberg, A.; Rabe, M.; Aigner, A. Multiple effects of the special AT-rich binding protein 1 (SATB1) in colon carcinoma. Int. J. cancer 2014, 135, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yang, C.; Wang, J.; Li, W.; Wen, R.; Chen, J.; Zheng, J. SATB1 is overexpressed in metastatic prostate cancer and promotes prostate cancer cell growth and invasion. J. Transl. Med. 2013, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Luo, M.; Wang, Z.; Yan, W.; Xia, Y.; Deng, H.; He, J.; Han, P.; Tian, D. Upregulation of SATB1 promotes tumor growth and metastasis in liver cancer. Liver Int. 2012, 32, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Nodin, B.; Hedner, C.; Uhlén, M.; Jirström, K. Expression of the global regulator SATB1 is an independent factor of poor prognosis in high grade epithelial ovarian cancer. J. Ovarian Res. 2012, 5, 24. [Google Scholar] [CrossRef]
- Nüssing, S.; Koay, H.-F.; Sant, S.; Loudovaris, T.; Mannering, S.I.; Lappas, M.; d′Udekem, Y.; Konstantinov, I.E.; Berzins, S.P.; Rimmelzwaan, G.F.; et al. Divergent SATB1 expression across human life span and tissue compartments. Immunol. Cell Biol. 2019. [Google Scholar] [CrossRef]
- Nodin, B.; Johannesson, H.; Wangefjord, S.; O’Connor, D.P.; Lindquist, K.E.; Uhlén, M.; Jirström, K.; Eberhard, J. Molecular correlates and prognostic significance of SATB1 expression in colorectal cancer. Diagn. Pathol. 2012, 7, 115. [Google Scholar] [CrossRef]
- Shukla, S.; Sharma, H.; Abbas, A.; MacLennan, G.T.; Fu, P.; Danielpour, D.; Gupta, S. Upregulation of SATB1 is associated with prostate cancer aggressiveness and disease progression. PLoS ONE 2013, 8, e53527. [Google Scholar] [CrossRef]
- Han, B.; Luan, L.; Xu, Z.; Wu, B. Expression and biological roles of SATB1 in human bladder cancer. Tumor Biol. 2013, 34, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, J.; Xiao, R.; Xu, G.; Liu, G.; Xiong, D.; Ye, Y.; Chen, B.; Wang, H.; Luo, Q.; et al. Poor prognosis and SATB1 overexpression in solid tumors: a meta-analysis. Cancer Manag. Res. 2018, 10, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Fu, X.; Li, Y.; Pang, X.; Chen, S.; Zhu, X.; Li, F.; Tan, W. SATB1 promotes epithelial-mesenchymal transition and metastasis in prostate cancer. Oncol. Lett. 2017, 13, 2577–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, J.H.; Wang, F.; Shen, M.H.; Wang, X.; Zhou, X.J.; Zhou, X.J.; Wang, Y.; Shen, M.H.; Wang, X.; Zhou, X.J.; et al. SATB1 expression is correlated with betha-catenin associated epithelial-mesenchymal transition in colorectal cancer. Cancer Biol. Ther. 2016, 17, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhou, L.; Li, S.; Xi, X.; Zhang, J.; Wang, Y.; Yang, Y.; Liu, X.; Wan, X. AT-rich sequence binding protein 1: Contribution to tumor progression and metastasis of human ovarian carcinoma. Oncol. Lett. 2012, 3, 865–870. [Google Scholar] [PubMed]
- Huang, B.O.; Zhou, H.; Wang, S.; Lang, X.P.; Wang, X. Effect of silencing SATB1 on proliferation, invasion and apoptosis of A549 human lung adenocarcinoma cells. Oncol. Lett. 2016, 12, 3818–3824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, B.; Zhang, X.; Sun, Y.; Wei, X.; McNutt, M.A.; Lu, S.; Liu, Y.; Zhang, D.; Wang, M.; et al. SATB1 expression is associated with biologic behavior in colorectal carcinoma in vitro and in vivo. PLoS ONE 2013, 8, e47902. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, X.; Ma, Y.; Jiang, J.; Dai, Z.; Yin, X.; Min, W.; Hui, W.; Wang, B. Expression and significance of SATB1 in the development of breast cancer. Genet. Mol. Res. 2015, 14, 3309–3317. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Qiao, C.; Qv, F.; Wang, J.; Ding, B.; Sun, Y.; Wang, Y. Expression of SATB1 and HER2 in breast cancer and the correlations with clinicopathologic characteristics. Diagn. Pathol. 2015, 10, 50. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Wang, Q.; Lu, Y.; Chen, H. Expression and clinical significance of SATB1 and TLR4 in breast cancer. Oncol. Lett. 2017, 14, 3611–3615. [Google Scholar] [CrossRef]
- Kobierzycki, C.; Wojnar, A.; Dziegiel, P. Expression of SATB1 Protein in the Ductal Breast Carcinoma Tissue Microarrays-Preliminary Study. Available online: https://journals.viamedica.pl/folia_histochemica_cytobiologica/article/view/FHC.2013.0045/26472 (accessed on 15 April 2016).
- van’t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.M.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iorns, E.; Hnatyszyn, H.J.; Seo, P.; Clarke, J.; Ward, T.; Lippman, M.; Kohwi-Shigematsu, T.; Han, H.-J.; Russo, J.; Kohwi, Y.; et al. The role of SATB1 in breast cancer pathogenesis. J. Natl. Cancer Inst. 2010, 102, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Jing, W.; He, K.; Zhang, L.; Long, X. SATB1 is Correlated with Progression and Metastasis of Breast Cancers: A Meta-Analysis. Cell. Physiol. Biochem. 2016, 38, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Kohwi-Shigematsu, T.; Han, H.-J.; Russo, J.; Kohwi, Y. Re: The role of SATB1 in breast cancer pathogenesis. J. Natl. Cancer Inst. 2010, 102, 1879–1880, author reply 1880–1881. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, C.; Zou, X.; Jiang, G.; Xu, Z.; Li, W.; Xie, H. Special AT-rich sequence-binding protein-1 participates in the maintenance of breast cancer stem cells through regulation of the Notch signaling pathway and expression of Snail1 and Twist1. Mol. Med. Rep. 2015, 11, 3235–3542. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, W.; Dai, Z.; Gao, X.; Ma, Y.; Xu, Q.; Jiang, J.; Zhang, S. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des. Devel. Ther. 2016, 10, 1419–1441. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-Y.; Xue, X.-H.; Ma, Y.-N.; Zhang, S.-Q. Effect of baicalein on the expression of SATB1 in human breast cancer cells. Exp. Ther. Med. 2015, 9, 1665–1669. [Google Scholar] [CrossRef]
- Patani, N.; Jiang, W.; Mansel, R.; Newbold, R.; Mokbel, K. The mRNA expression of SATB1 and SATB2 in human breast cancer. Cancer Cell Int. 2009, 9, 18. [Google Scholar] [CrossRef]
- Hanker, L.C.; Karn, T.; Mavrova-Risteska, L.; Ruckhäberle, E.; Gaetje, R.; Holtrich, U.; Kaufmann, M.; Rody, A.; Wiegratz, I. SATB1 gene expression and breast cancer prognosis. The Breast 2011, 20, 309–313. [Google Scholar] [CrossRef]
- Laurinavicius, A.; Green, A.R.; Laurinaviciene, A.; Smailyte, G.; Ostapenko, V.; Meskauskas, R.; Ellis, I.O. Ki67/SATB1 ratio is an independent prognostic factor of overall survival in patients with early hormone receptor-positive invasive ductal breast carcinoma. Oncotarget 2015, 6, 41134–41145. [Google Scholar] [CrossRef]
- Kobierzycki, C.; Grzegrzolka, J.; Glatzel-Plucinska, N.; Piotrowska, A.; Wojnar, A.; Smolarz, B.; Romanowicz, H.; Dziegiel, P. Expression of p16 and SATB1 in Invasive Ductal Breast Cancer—A Preliminary Study. In Vivo 2018, 32, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, A.; Berx, G. Cadherins and Epithelial-to-Mesenchymal Transition. Prog. Mol. Biol. Transl. Sci. 2013, 116, 317–336. [Google Scholar] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yang, C.; Fan, L.; Gao, P.; Yang, D.; Xue, B.; Zheng, J.; Shan, Y. SATB1 promotes prostate cancer metastasis by the regulation of epithelial–mesenchymal transition. Biomed. Pharmacother. 2016, 79, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Cheng, C.; Wang, Z.; Xiao, X.; Zeng, H.; Xing, S.; Chen, X.; Wang, J.; Li, S.; Zhang, Y.; et al. SATB1 overexpression regulates the development and progression in bladder cancer through EMT. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Radice, G.L. N-cadherin-mediated adhesion and signaling from development to disease: lessons from mice. Prog. Mol. Biol. Transl. Sci. 2013, 116, 263–289. [Google Scholar] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Dechat, T.; Grin, B.; Helfand, B.; Mendez, M.; Pallari, H.-M.; Goldman, R.D. Introducing intermediate filaments: from discovery to disease. J. Clin. Invest. 2009, 119, 1763–1771. [Google Scholar] [CrossRef] [Green Version]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccin. Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.-Y.; Yang, P.-C. The EMT regulator slug and lung carcinogenesis. Carcinogenesis 2011, 32, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Medici, D.; Hay, E.D.; Olsen, B.R. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol. Biol. Cell 2008, 19, 4875–4887. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-Q.; Ma, C.; Wang, Q.; Song, Y.; Lv, T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumor Biol. 2016, 37, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Chen, H.; Zhang, D.; Fu, J. Twist: a molecular target in cancer therapeutics. Tumor Biol. 2013, 34, 2497–2506. [Google Scholar] [CrossRef]
- Ubink, I.; Verhaar, E.R.; Kranenburg, O.; Goldschmeding, R. A potential role for CCN2/CTGF in aggressive colorectal cancer. J. Cell Commun. Signal. 2016, 10, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-S.; Wang, M.-Y.; Wu, S.-N.; Su, J.-L.; Hong, C.-C.; Chuang, S.-E.; Chen, M.-W.; Hua, K.-T.; Wu, Y.-L.; Cha, S.-T.; et al. CTGF enhances the motility of breast cancer cells via an integrin-alphavbeta3-ERK1/2-dependent S100A4-upregulated pathway. J. Cell Sci. 2007, 120, 2053–2065. [Google Scholar] [CrossRef]
- Erlandsson, M.C.; Svensson, M.D.; Jonsson, I.-M.; Bian, L.; Ambartsumian, N.; Andersson, S.; Peng, Z.; Vääräniemi, J.; Ohlsson, C.; Andersson, K.M.E.; et al. Expression of metastasin S100A4 is essential for bone resorption and regulates osteoclast function. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 2653–2663. [Google Scholar] [CrossRef] [Green Version]
- ZHENG, X.; GAI, X.; WU, Z.; LIU, Q.; YAO, Y. Metastasin leads to poor prognosis of hepatocellular carcinoma through partly inducing EMT. Oncol. Rep. 2013, 29, 1811–1818. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 6152–6157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart; WHO: Lyon, France, 2015. [Google Scholar]
- Zhou, L.-Y.; Liu, F.; Tong, J.; Chen, Q.-Q.; Zhang, F.-W. Expression of special AT-rich sequence-binding protein mRNA and its clinicopathological significance in non-small cell lung cancer. J. South. Med. Univ. 2009, 29, 534–537. [Google Scholar]
- Beer, D.G.; Kardia, S.L.R.R.; Huang, C.-C.C.-C.; Giordano, T.J.; Levin, A.M.; Misek, D.E.; Lin, L.; Chen, G.; Gharib, T.G.; Thomas, D.G.; et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat. Med. 2002, 8, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Stearman, R.S.; Dwyer-Nield, L.; Zerbe, L.; Blaine, S.A.; Chan, Z.; Bunn, P.A.; Johnson, G.L.; Hirsch, F.R.; Merrick, D.T.; Franklin, W.A.; et al. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol 2005, 167, 1763–1775. [Google Scholar] [CrossRef]
- Talbot, S.G.; Estilo, C.; Maghami, E.; Sarkaria, I.S.; Pham, D.K.; O-charoenrat, P.; Socci, N.D.; Ngai, I.; Carlson, D.; Ghossein, R.; et al. Gene expression profiling allows distinction between primary and metastatic squamous cell carcinomas in the lung. Cancer Res. 2005, 65, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Glatzel-Plucinska, N.; Piotrowska, A.; Grzegrzolka, J.; Olbromski, M.; Rzechonek, A.; Dziegiel, P.; Podhorska-Okolow, M. SATB1 Level Correlates with Ki-67 Expression and Is a Positive Prognostic Factor in Non-small Cell Lung Carcinoma. Anticancer Res. 2018, 38, 723–736. [Google Scholar] [PubMed]
- Selinger, C.I.; Cooper, W.A.; Al-Sohaily, S.; Mladenova, D.N.; Pangon, L.; Kennedy, C.W.; McCaughan, B.C.; Stirzaker, C.; Kohonen-Corish, M.R.J. Loss of special AT-rich binding protein 1 expression is a marker of poor survival in lung cancer. J. Thorac. Oncol. 2011, 6, 1179–1189. [Google Scholar] [CrossRef]
- Huang, B.; Zhou, H.; Wang, X.; Liu, Z. Silencing SATB1 with siRNA inhibits the proliferation and invasion of small cell lung cancer cells. Cancer Cell Int. 2013, 13, 8. [Google Scholar] [CrossRef]
- Meng, W.-J.; Yan, H.; Zhou, B.; Zhang, W.; Kong, X.-H.; Wang, R.; Zhan, L.; Li, Y.; Zhou, Z.-G.; Sun, X.-F. Correlation of SATB1 overexpression with the progression of human rectal cancer. Int. J. Colorectal Dis. 2012, 27, 143–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Ji, H.; Guan, X.; Xu, W.; Dong, B.; Zhao, M.; Wei, M.; Ye, C.; Sun, Y.; et al. Expression of SATB1 promotes the growth and metastasis of colorectal cancer. PLoS ONE 2014, 9, e100413. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, L.; Cheng, C.; Du, C.; Lu, X.; Wang, G.; Liu, J. Increased expressions of SATB1 and S100A4 are associated with poor prognosis in human colorectal carcinoma. APMIS 2015, 123, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.E.; Godlewski, J.; Krazinski, B.E.; Kiewisz, J.; Sliwinska-Jewsiewicka, A.; Kwiatkowski, P.; Pula, B.; Dziegiel, P.; Janiszewski, J.; Wierzbicki, P.M.; et al. Divergent expression patterns of SATB1 mRNA and SATB1 protein in colorectal cancer and normal tissues. Tumour Biol. 2015, 36, 4441–4452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, H.; Ishikawa, T.; Mogushi, K.; Ishiguro, M.; Uetake, H.; Tanaka, H.; Sugihara, K. Identification of SATB1 as a Specific Biomarker for Lymph Node Metastasis in Colorectal Cancer. Anticancer Res. 2016, 36, 4069–4076. [Google Scholar] [PubMed]
- Mansour, M.A.; Hyodo, T.; Akter, K.A.; Kokuryo, T.; Uehara, K.; Nagino, M.; Senga, T. SATB1 and SATB2 play opposing roles in c-Myc expression and progression of colorectal cancer. Oncotarget 2016, 7, 4993–5006. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.-J.; Pathak, S.; Ding, Z.-Y.; Zhang, H.; Adell, G.; Holmlund, B.; Li, Y.; Zhou, Z.-G.; Sun, X.-F. Special AT-rich sequence binding protein 1 expression correlates with response to preoperative radiotherapy and clinical outcome in rectal cancer. Cancer Biol. Ther. 2015, 16, 1738–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sohaily, S.; Henderson, C.; Selinger, C.; Pangon, L.; Segelov, E.; Kohonen-Corish, M.R.J.; Warusavitarne, J. Loss of special AT-rich sequence-binding protein 1 (SATB1) predicts poor survival in patients with colorectal cancer. Histopathology 2014, 65, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Pradhan, S.J.; Patil, P.; Mulherkar, R.; Galande, S. Wnt/β-catenin signaling regulated SATB1 promotes colorectal cancer tumorigenesis and progression. Oncogene 2016, 35, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qian, J.; Wang, F.; Ma, Z. Cellular prion protein accelerates colorectal cancer metastasis via the Fyn-SP1-SATB1 axis. Oncol. Rep. 2012, 28, 2029–2034. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Tuo, Y.; Luo, W.; He, S.; Chen, Y. Prognostic and Clinicopathological Significance of SATB1 in Colorectal Cancer: A Meta-Analysis. Front. Physiol. 2018, 9, 535. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Zhang, J.; Liu, N.; Fan, L.; Yang, D.; Xue, B.; Shan, Y.; ZHENG, J. Oncolytic virus carrying shRNA targeting SATB1 inhibits prostate cancer growth and metastasis. Tumor Biol. 2015, 36, 9073–9081. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, S.-C.; Yang, C.-S.; Chen, J.-C.; Zheng, J.-N.; Sun, X.-Q.; Wang, J.-Q. Inhibition of prostate cancer cell growth in vivo with short hairpin RNA targeting SATB1. Oncol. Lett. 2017, 14, 6592–6596. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cheng, C.; Zhu, S.; Yang, Y.; Zheng, L.; Wang, G.; Shu, X.; Wu, K.; Liu, K.; Tong, Q. SATB1 is an independent prognostic marker for gastric cancer in a Chinese population. Oncol. Rep. 2010, 24, 981–987. [Google Scholar] [PubMed] [Green Version]
- Cheng, C.; Lu, X.; Wang, G.; Zheng, L.; Shu, X.; Zhu, S.; Liu, K.; Wu, K.; Tong, Q. Expression of SATB1 and heparanase in gastric cancer and its relationship to clinicopathologic features. APMIS 2010, 118, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-L.; Li, L.; Zhou, X.; Li, H.; Han, L. Expression of SATB1 and HER2 in gastric cancer and its clinical significance. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2256–2264. [Google Scholar] [PubMed]
- Yang, L.; Zheng, R.; Wang, N.; Yuan, Y.; Liu, S.; Li, H.; Zhang, S.; Zeng, H.; Chen, W. Incidence and mortality of stomach cancer in China, 2014. Chin. J. Cancer Res. 2018, 30, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, C.; Fang, E.; Lu, X.; Wang, G.; Tong, Q. Co-delivery of doxorubicin and SATB1 shRNA by thermosensitive magnetic cationic liposomes for gastric cancer therapy. PLoS ONE 2014, 9, e92924. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, Z.; Zheng, L.; Qin, J.; Li, H.; Xue, X.; Gao, J.; Fang, G. SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells. Onco. Targets. Ther. 2018, 11, 6811–6825. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.-X.; Zhang, H.; Sun, S.-X.; Li, H.-F.; Wang, Y.; Jian, S. Pilot study special AT-rich sequence-binding protein 1 investigating as a potential biomarker for esophageal squamous cell carcinoma. Dis. Esophagus 2016, 29, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liu, K.; Yang, X.; Mu, B.; Yang, J.; He, L.; Hu, X.; Li, Q.; Zhao, Y.; Cai, X.; et al. SATB1 plays an oncogenic role in esophageal cancer by up-regulation of FN1 and PDGFRB. Oncotarget 2017, 8, 17771–17784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Tong, Y.X.; Xu, X.S.; Lin, H.; Chao, T.F. Prognostic significance of SATB1 in gastrointestinal cancer: a meta-analysis and literature review. Oncotarget 2017, 8, 48410–48423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedner, C.; Gaber, A.; Korkocic, D.; Nodin, B.; Uhlén, M.; Kuteeva, E.; Johannesson, H.; Jirström, K.; Eberhard, J. SATB1 is an independent prognostic factor in radically resected upper gastrointestinal tract adenocarcinoma. Virchows Arch. 2014, 465, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, J.; Wu, K.; Chen, L.; Yu, J.; Hu, W.; Zhang, K. SATB1 is a potential therapeutic target in intrahepatic cholangiocarcinoma. Clin. Transl. Oncol. 2016, 18, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, D.; Li, X.; Zhong, K.; Liu, X.; Bi, M.; Liu, Y.; Liao, X.; Lin, L. Expression of SATB1 in hepatocellular carcinoma cell lines with different invasive capacities. J. South. Med. Univ. 2012, 32, 994. [Google Scholar]
- Chen, Z.; Li, Z.; Li, W.; Zong, Y.; Zhu, Y.; Miao, Y.; Xu, Z. SATB1 Promotes Pancreatic Cancer Growth and Invasion Depending on MYC Activation. Dig. Dis. Sci. 2015, 60, 3304–3317. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zheng, J.; Yu, T.; Liu, Y.; Duo, L. Elevated expression of SATB1 is involved in pancreatic tumorigenesis and is associated with poor patient survival. Mol. Med. Rep. 2017, 16, 8842–8848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elebro, J.; Heby, M.; Gaber, A.; Nodin, B.; Jonsson, L.; Fristedt, R.; Uhlén, M.; Jirström, K.; Eberhard, J. Prognostic and treatment predictive significance of SATB1 and SATB2 expression in pancreatic and periampullary adenocarcinoma. J. Transl. Med. 2014, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Clement, J.M.; Choudhary, S.; Voznesensky, O.; Pilbeam, C.C.; Woolbright, B.L.; Taylor, J.A. SATB1 and bladder cancer: Is there a functional link? Urol. Oncol. Semin. Orig. Investig. 2018, 36, 93.e13–93.e21. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Wang, Q. Expression of SATB1 and E-cad in tissues of patients with endometrial carcinoma and the relationship with clinicopathological features. Exp. Ther. Med. 2018, 15, 4339–4343. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, L.; Liu, Y.; Meng, F.; Wang, S.; Shang, P.; Gao, Y.; Chen, X. Overexpression of Special AT-Rich Sequence-Binding Protein 1 in Endometrial Cancer. Int. J. Gynecol. Cancer 2015, 25, 4–11. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Zhang, Y.; Liu, Y.; Meng, F.; Ma, J.; Shang, P.; Gao, Y.; Huang, Q.; Chen, X. Special AT-rich sequence-binding protein 1: a novel biomarker predicting cervical squamous cell carcinoma prognosis and lymph node metastasis. Jpn. J. Clin. Oncol. 2015, 45, 812–818. [Google Scholar] [CrossRef]
- Meng, W.-J.; Yan, H.; Li, Y.; Zhou, Z.-G. SATB1 and colorectal cancer in Wnt/β-catenin signaling: Is there a functional link? Med. Hypotheses 2011, 76, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayana Reddy, C.N.; Vyjayanti, V.N.; Notani, D.; Galande, S.; Kotamraju, S. Down-regulation of the global regulator SATB1 by statins in COLO205 colon cancer cells. Mol. Med. Rep. 2010, 3, 857–861. [Google Scholar] [PubMed]
- Wang, M.; Yin, B.; Matsueda, S.; Deng, L.; Li, Y.; Zhao, W.; Zou, J.; Li, Q.; Loo, C.; Wang, R.-F.; et al. Identification of special AT-rich sequence binding protein 1 as a novel tumor antigen recognized by CD8+ T cells: implication for cancer immunotherapy. PLoS ONE 2013, 8, e56730. [Google Scholar] [CrossRef] [PubMed]

| Category | Gene Symbol | Protein Name | Function | Effect of Modulation by SATB1 | Malignancy |
|---|---|---|---|---|---|
| Epithelial Markers | CDH1 | E-cadherin | Cell adhesion molecule, responsible for maintaining epithelial integrity [66]. Downregulation of E-cadherin, resulting in destabilization of adherens junctions, is one of the main hallmarks of EMT [67]. | ↓ Downregulation | Breast cancer [17] Colorectal cancer [37] Prostate cancer [43,46,68] Liver cancer [39] Bladder cancer [69] |
| CLDN1 | Claudin 1 | Tight junctions protein that regulates the permeability of epithelia. During EMT, its expression is downregulated as a result of SNAIL, SLUG and Twist1 activity [67]. | ↓ Downregulation | Breast cancer [17] | |
| Mesenchymal Markers | CDH2 | N-cadherin | Cell adhesion molecule, taking part in various cellular processes, including proliferation, migration and apoptosis [70]. The gain of N-cadherin expression is thought to be a critical step in epithelial cancer metastasis [71]. | ↑ Upregulation | Breast cancer [17] Colorectal cancer [37] |
| VIM | Vimentin | Intermediate filaments protein, expressed in mesenchymal cells. It maintains cell integrity and flexibility [72]. | ↑ Upregulation | Breast cancer [17] Prostate cancer [46,68] Liver cancer [39] Bladder cancer [69] | |
| MMP-2, MMP-7, MMP-9 | Matrix metalloproteinases (MMPs) | A family of proteases that digest components of the extracellular matrix. MMPs not only allow cell migration, but can also contribute to EMT by activating TGFβ [73]. | ↑ Upregulation | Breast cancer [17] Colorectal cancer [37] Prostate cancer [43,68] | |
| EMT Inducers | TGFB | Transforming Growth Factor β (TGF-β) | TGF-β is a multi-functional cytokine that regulates cell growth and differentiation, apoptosis, and cell motility [74]. TGF- β signalling has also been shown to play an important role in EMT induction [75]. | ↑ Upregulation | Breast cancer [17] |
| SNAI1 | SNAIL | Proteins belonging to the SNAIL superfamily of zinc-finger transcription factors. They repress E-cadherin expression and act as critical regulators of multiple pathways leading to EMT [76,77,78]. | ↑ Upregulation | Breast cancer [59] Liver cancer [39] Bladder cancer [69] | |
| SNAI2 | SLUG | ↑ Upregulation | Colorectal cancer [37] Liver cancer [39] Bladder cancer [69] | ||
| Twist1 | Twist1 | TWIST1 is a basic helix-loop-helix (bHLH) transcription factor that plays a role of one of the most important EMT regulators [79,80]. It decreases E-cadherin, and increases N-cadherin expression. Furthermore, Twist1 can contribute to metastasis due to its pro-angiogenic and anti-apoptotic functions [80]. | ↑ Upregulation | Breast cancer [59] Colorectal cancer [37] Liver cancer [39] | |
| Other Factors | CCN2 | Connective Tissue Growth Factor (CTGF) | CTGF plays role in cells’ proliferation, adhesion, migration, and angiogenesis. It was also demonstrated to mediate tumorigenesis and increase metastatic potential of the cells [81,82]. | ↑ Upregulation | Breast cancer [17] |
| S100A4 | Metastasin | Ca-binding protein that regulates cell growth, survival and motility [83]. It was shown to increase cells’ migration and invasion capacities, and to be associated with tumour metastasis [84]. | ↑ Upregulation | Breast cancer [17] | |
| VEGF-B | VEGF B | A growth factor belonging to the vascular endothelial growth factor family. VEGF B is responsible for maintaining newly formed blood vessels, especially those developed under pathological conditions [85]. | ↑ Upregulation | Breast cancer [17] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glatzel-Plucińska, N.; Piotrowska, A.; Dzięgiel, P.; Podhorska-Okołów, M. The Role of SATB1 in Tumour Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 4156. https://doi.org/10.3390/ijms20174156
Glatzel-Plucińska N, Piotrowska A, Dzięgiel P, Podhorska-Okołów M. The Role of SATB1 in Tumour Progression and Metastasis. International Journal of Molecular Sciences. 2019; 20(17):4156. https://doi.org/10.3390/ijms20174156
Chicago/Turabian StyleGlatzel-Plucińska, Natalia, Aleksandra Piotrowska, Piotr Dzięgiel, and Marzenna Podhorska-Okołów. 2019. "The Role of SATB1 in Tumour Progression and Metastasis" International Journal of Molecular Sciences 20, no. 17: 4156. https://doi.org/10.3390/ijms20174156